Are you a healthcare professional?👨⚕️🏥👩⚕️
Adakah anda seorang anggota kesihatan? 👨⚕️🏥👩⚕️

You can help us make medicines safer for everyone
👉 by reporting any new side effects that your patients are experiencing to the NPRA ADR/AEFI Reporting System.
Anda boleh membantu kami menjadikan ubat-ubatan lebih selamat untuk semua
👉 dengan melaporkan sebarang kesan sampingan baharu yang dialami oleh pesakit anda kepada Sistem Pelaporan ADR/AEFI NPRA.
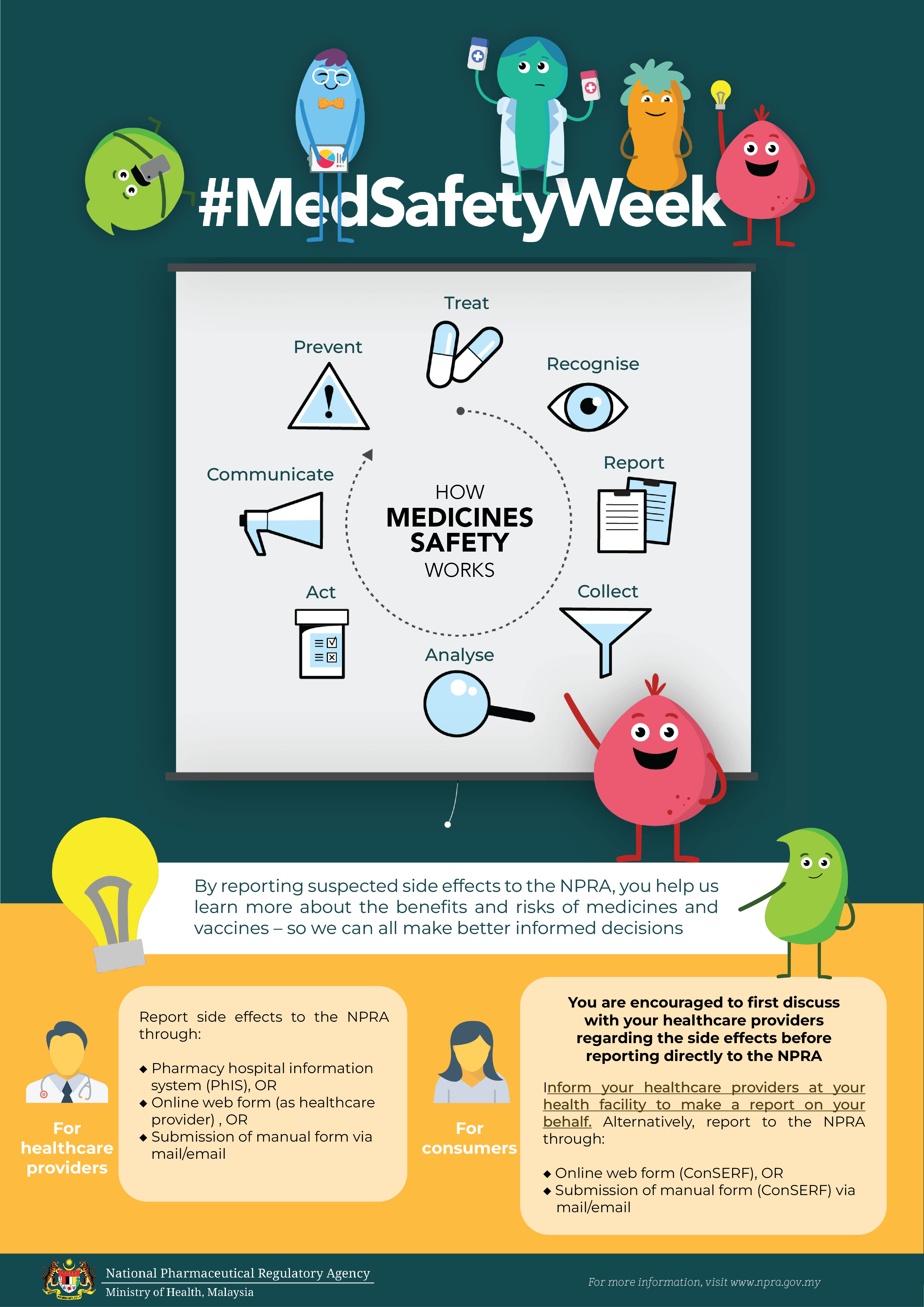
How MEDICATION SAFETY works?
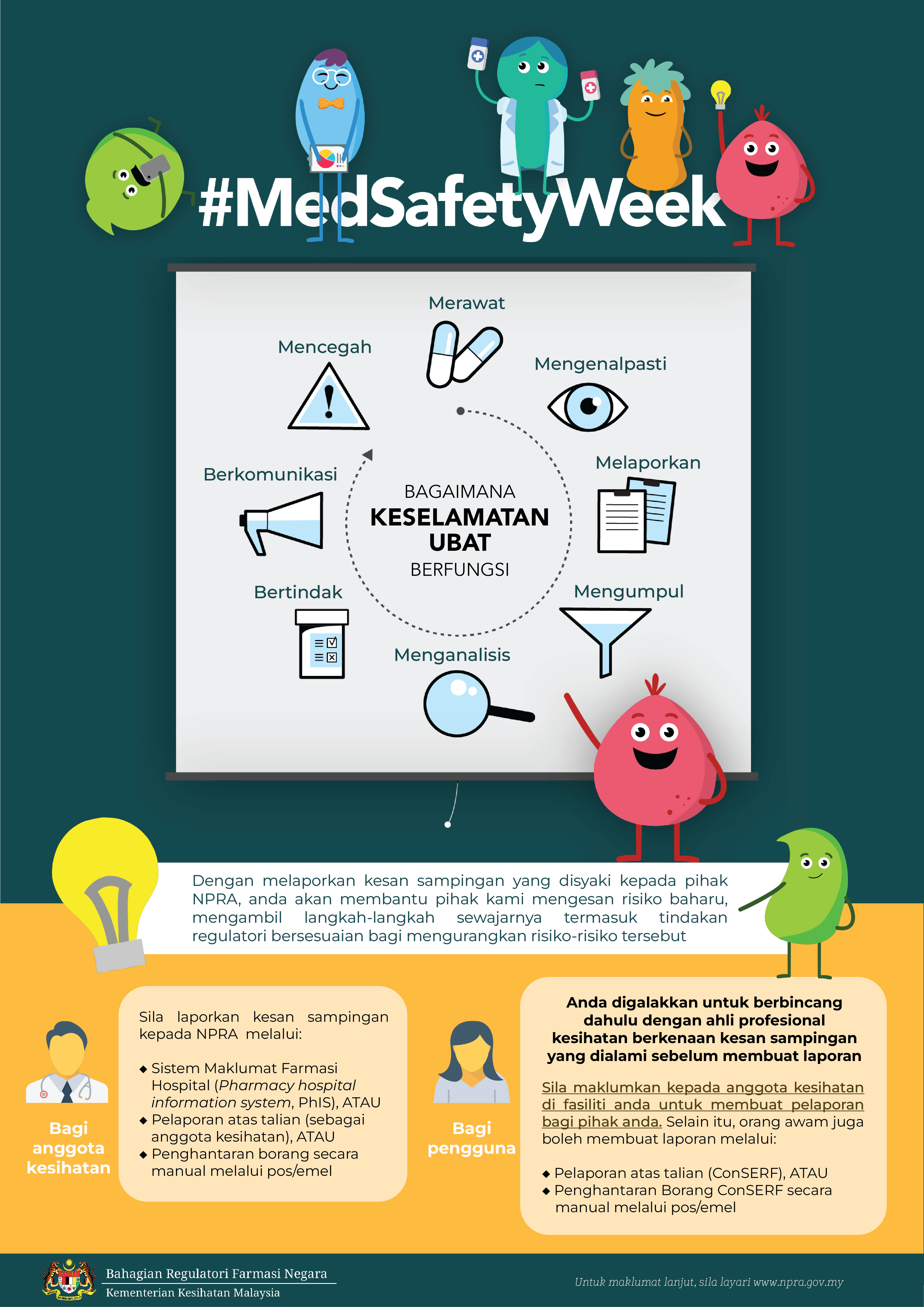
Bagaimana KESELAMATAN UBAT berfungsi?
Side effects that are not already in the Package Insert or Consumer Medication Information Leaflet (RiMUP) need to be reported immediately,
so we can investigate and take any necessary actions.
Kesan sampingan yang belum ada dalam Sisip Bungkusan atau Risalah Maklumat Ubat Untuk Pengguna (RiMUP) perlu dilaporkan dengan segera
supaya dapat disiasat dan diambil tindakan sewajarnya.
Always stay up to date with new information about side effects and medicines,
so you can advise the best treatment for your patients and improve #PatientSafety.
Sentiasa ikuti perkembangan terkini keselamatan ubat-ubatan dan dapatkan maklumat terbaharu mengenai kesan sampingan
supaya anda boleh memberikan rawatan yang terbaik dan meningkatkan keselamatan pesakit anda #PatientSafety.
#MedSafetyWeek #ReportSideEffects #Pharmacovigilance








