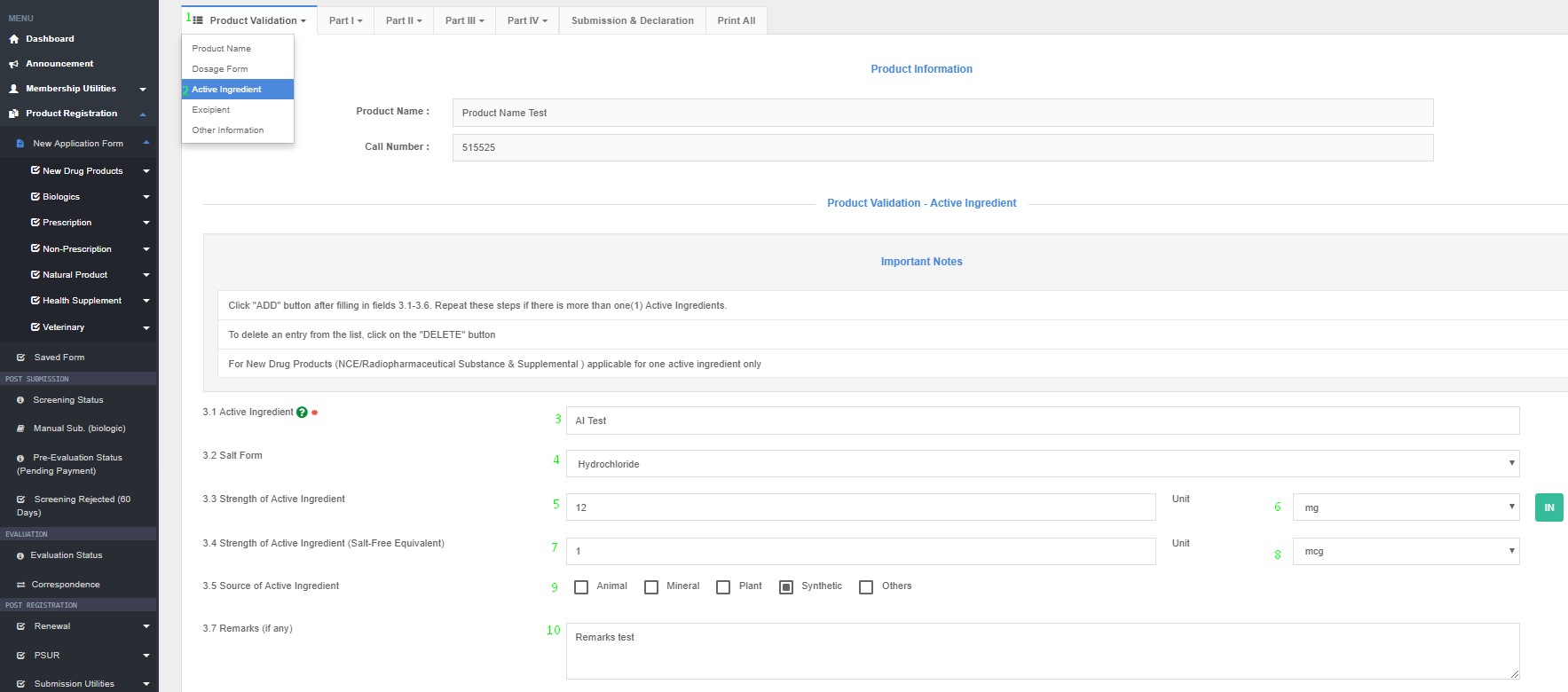
The actual raw material that is employed in the manufacturing process shall be named. For example:
- Where the raw material used is the salt (e.g. ampicillin trihydrate) which will yield an equivalent effective component from its base content (i.e. ampicillin), the substance name is the salt and the equivalent base component should be indicated in remarks on substance field, if any.
Please refer:
Drug Registration Guidance Document (DRGD) - Appendix 8.1 List of Prohibited and Restricted Active Ingredients and Combinations.
How to Access Active Ingredient in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Active Ingredients