Frequently Asked Questions (FAQs) : Active Pharmaceutical Ingredient (API)
A) API in New Product Applications
B) API in Registered Products (Unregulated API)
(Product Registered Before the Implementation of Directive on Regulatory Control of API in Malaysia)
i) Category of Product: Anti-Infectives
Section S revision shall be submitted to New Drug Product Section or Generic Medicine Section 12 to 15 months prior to expiry of product registration period. Any submissions other than the indicated period will not be accepted.
"Section S Revision" is the terminology used for the administrative procedure for the implementation of regulatory control of API in registered product exipiring starting 1 Jan 2020.
"Product Editing" is the sub-module in QUEST3+ that enables updating of API information with the exercise of Section S Revision.
No. Any submissions other than the indicated period will not be accepted.
Once applicant has completed the online form and click the “submit” button, a pop-up notification will be received indicating a successful submission. In addition, a copy of the submitted form will be emailed to the recipient of email address entered in Form RegA2.
Within 15 calendar days. Please refer to schedule of enabling Section S Revision in Quest 3+.
Please refer to summary of required documents in Guidance Note for API Information: Appendix 1: API submission Checklist.
The regulatory control of API is based on product category. This kind of product will be classified as anti-infective. Thus, both APIs should be submitted through single procedure and should follow the same requirement i.e. Anti-Infective
Please refer to the ATC code for confirmation.
Yes. Applicant can submit variation(s) application after submitting “Product Editing” in QUEST system. Please also refer to Answer No.9, Quest 3+ system cannot support more than one process at one time (i.e. variation/evaluation/section S revision/renewal etc.). Thus, applicants shall plan the submission for each process, so that it does not overlap.
No. If there are any changes in name and address of API manufacturer, or change of API specification, please submit a variation application. Please note that “Section S Revision” is not a platform to submit post-approval changes. Post-approval changes shall comply to Malaysian Variation Guidelines. Section S Revision is a procedure to allow submission of Part II S information for unregulated APIs as required by the Directive of Regulatory Control of API (BPFK/PPP/07/25(7)) dated 16 Jan 2014.
Applicants are only required to submit Form Reg A2 for the case mentioned. When submitting Form Reg A2, please fill in product information and “tick” the option of “No Changes Made Since Data Cleaning / Registration / Variation Approval” in Page 3 of Form Reg A2. After verification by NPRA officer, this product will be remarked as “fulfilled Section S Revision”. Therefore, “Product Editing” via QUEST system is not required.
Please refer to the procedure flow for Section S Revision at https://shorturl.at/W3SiY. Below is a brief description of the process flow:
- Prepare the required documents as stated in https://shorturl.at/NSc3X.
- Once you have the required information, submit Form RegA2 (https://shorturl.at/B3KVo) to request to perform Section S Revision. NPRA officer will process the applications (according to schedule at https://shorturl.at/4UdBl) and enable “Product Editing” in QUEST system.
- The applicant shall upload all required API information via “Product Editing” module in QUEST system and click “Submit”.
- After submission of “Product Editing”, the requirement for Section S Revision has been fulfilled for renewal procedure.
- NPRA officer will evaluate the API information provided. Any additional information required will be requested via email stated in Form Reg A2.
- NPRA officer will issue via email “Notification of Completion of Section S Revision” upon satisfactory review of the API information.
No. “Section S Revision” only need to be fulfilled once. Any post marketing changes shall comply to the Drug Registration Guidance Document and the Malaysian Variation Guideline.
ii) Category of Product: Non Anti-Infectives
It is Product Registration Holder's (PRH) responsibility to ensure that the API used in the finished product fulfills NPRA’s requirement. PRH is responsible to ensure that all required API information are available on hand. This information shall be kept safe with the PRH without uploading into QUEST3+. With this change, NPRA hopes to reduce administrative work at both ends. However, when required, NPRA reserve the right to request PRH to provide required API information within a given timeframe.
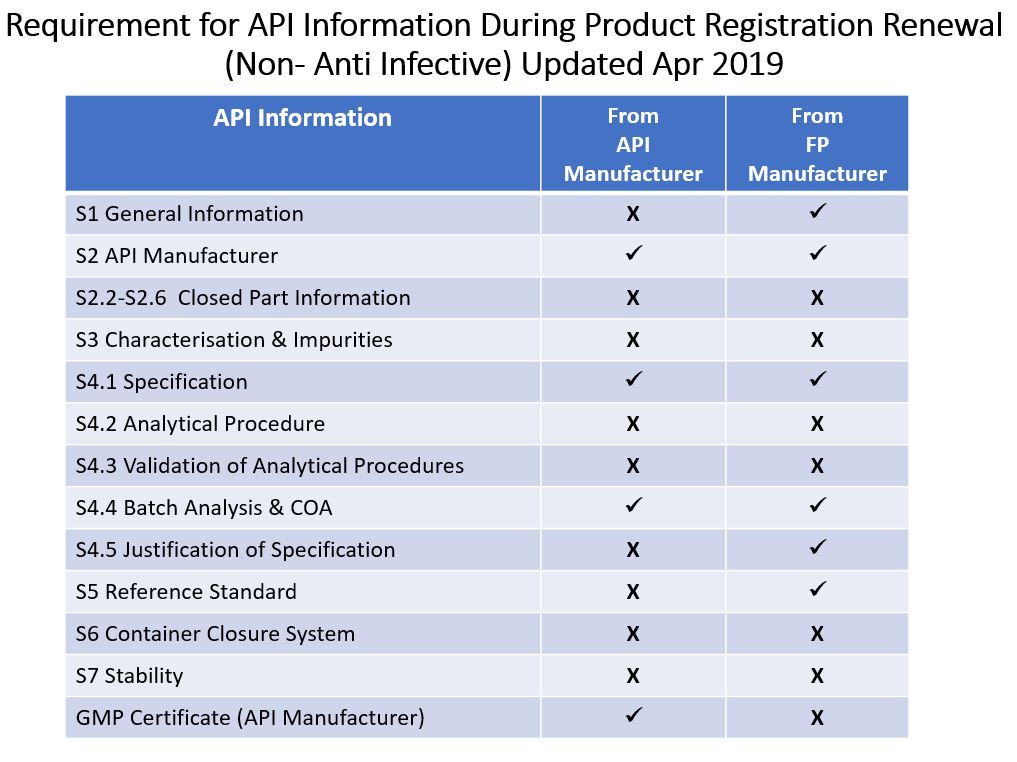
Foot Note:
X - Optional
/ - RequiredThe regulatory control of API is based on product category. This kind of products will be classified as anti-infectives. Thus, both APIs should be submitted with the requirement as stated in Guidance Note for API Information: Appendix 1.
No. The change of administrative procedure aims to reduce administrative workflow. "Product Editing" are strictly for Anti-Infective products undergoing Section S Revision only. Products that require “data cleaning” will not be entertained.







