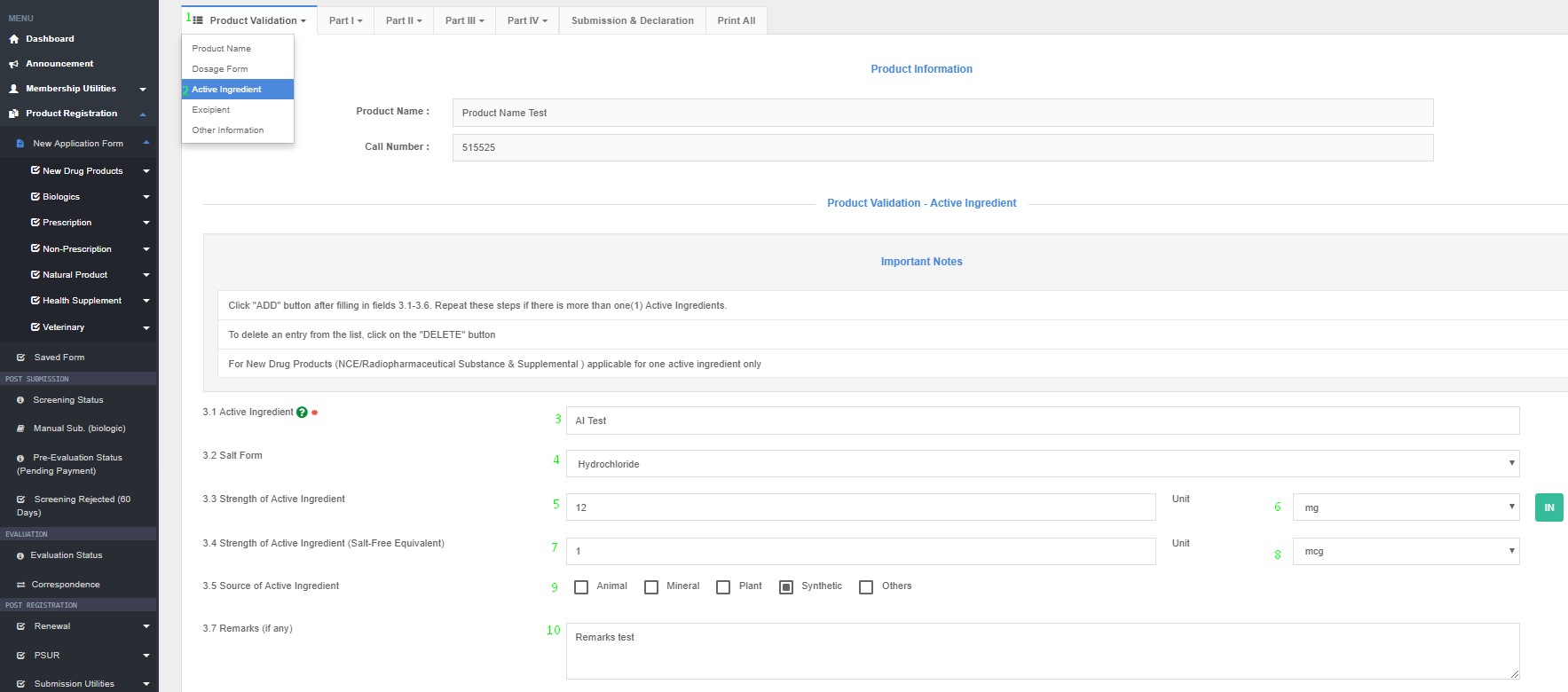
Active Ingredient(s)
3.1 Active Ingredient Name
3.2 Salt Form
3.3 Strength of Active Ingredient (Quantity unit/ dose)
3.4 Strength Salt-Free
3.5 Source of Active Ingredient (Animal – e.g. Bovine, Porcine, Ovine or Others/ Plant/ Others)
3.6 Form of Substance
3.7 Remarks (if any)
3.8 Status
- Listed active ingredients can be checked through http://npra.moh.gov.my/ of product search. Ingredients not listed will require safety and/or efficacy data evaluation prior to addition to this list.
- Substances that are included in the formulation as active ingredients must make a contribution to the proposed indications for the product.
- Please specify the source such as animal, plant, synthetic or others.
- Please check whether the product contains active ingredients listed in the PROTECTED/ ENDANGERED WILDLIFE OR BOTANICAL SPECIES as in the DRGD. The applicant shall contact the appointed department as listed in DRGD to obtain the necessary permit / license.
Strength of active ingredient :
- To enter the content of active ingredients (numerical) and then select the weights and measures from the given list.
- The content of ingredients shall be expressed as appropriate in the following manner:
- a) quantity per dose unit (e.g. for unit dose formulations - tablet, capsule, lozenge, etc.)
- b) percentage composition - %w/w, %w/v, %v/v, etc.
- c) weight per ml. (e.g. for solutions, suspension etc.)
- Quantity (percentage or amount) per measured dose (e.g. oral liquids, drops, etc.)
- Metric weights and measures shall be used.
How to Access Active Ingredient in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Active Ingredients