Please click "Details" button to open Section S for each API.
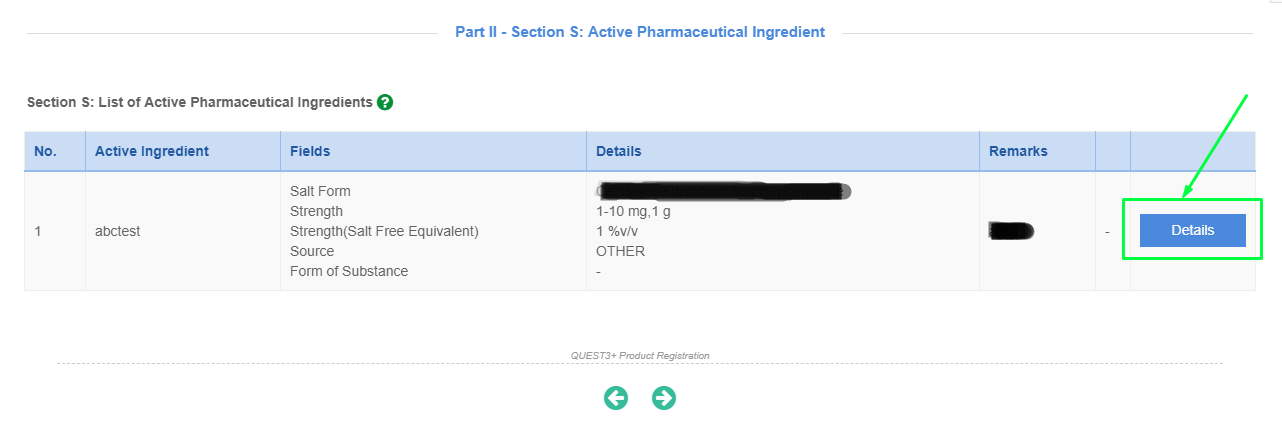
- "API Approved by NPRA" refers to API regulated and approved following the implementation of Directive on Regulatory Control of API in Malaysia dated 17 Mar 2011.
- "API Approved by NPRA" also refers to API previously reviewed and approved by the same PRH, with the same API manufacturer [with the same synthesis route].
*For API Approved by NPRA, please fill up Declaration Letter for an Approved API in New Product Registration Application and attach at later page under S10.
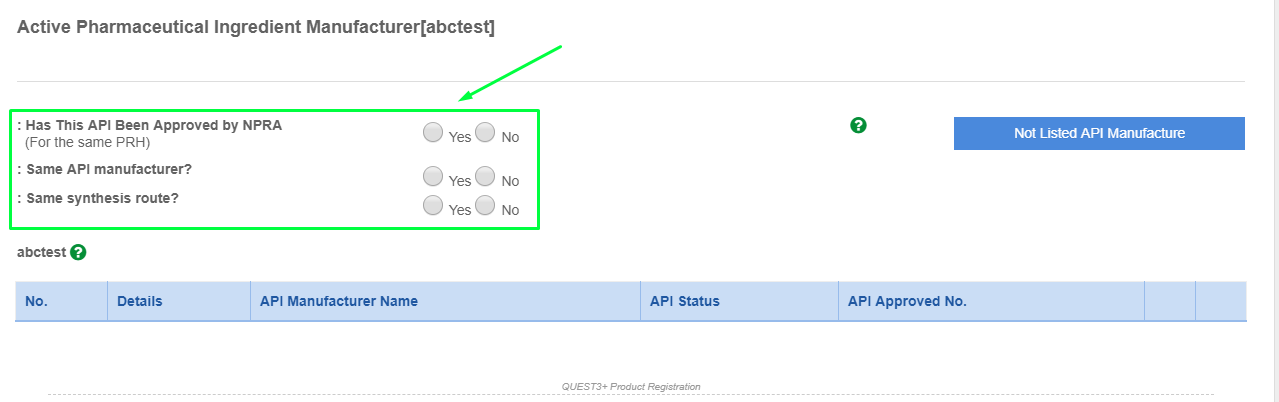
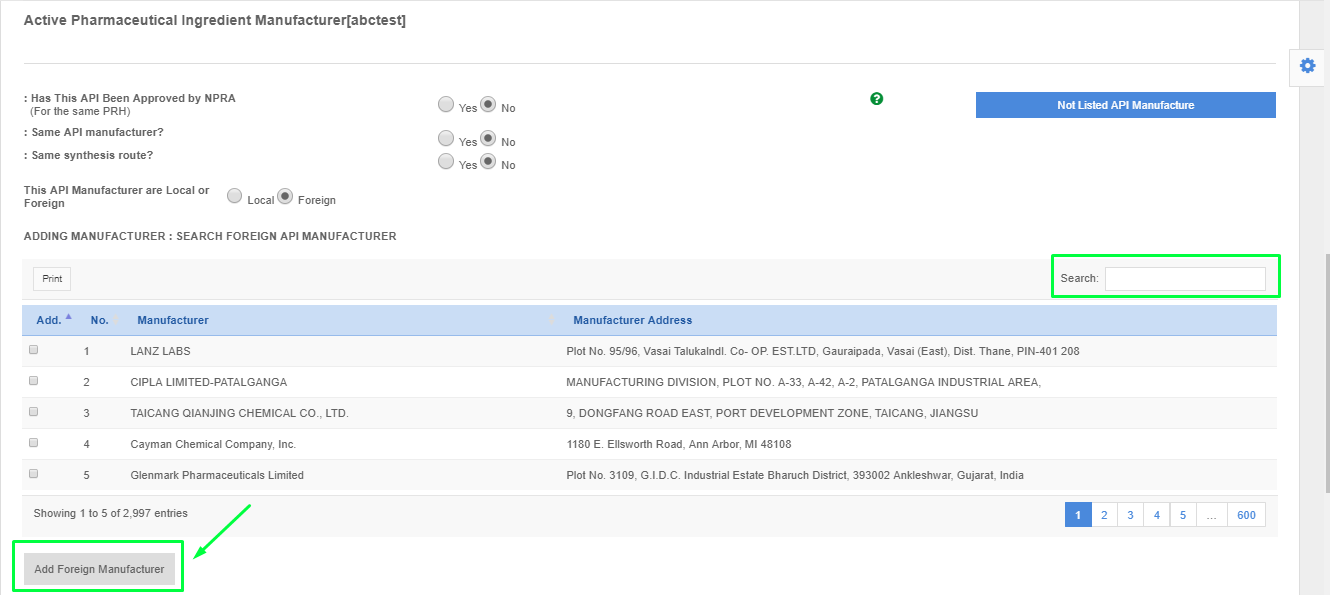
- Please find the API manufacturer using the search box.
- Please click the API manufacturer and click "Add Foreign API Manufacturer".
- If the API manufacturer is not found from the list. Please click "Not Listed API Manufacturer" and fill up the form to request for listing.
- For a finished product containing more than one API, the information requested for Part II S should be provided entirely for each API.
- Separate DMF/CEP/Part II S must be provided entirely for each API for:
- Finished product contains more than one API
- API from different manufacturing site
- API from different synthesis route
- CEP
- DMF
- ACTD
References:
Appendix 6 : Guideline On Regulatory Control Of Active Pharmaceutical Ingredients (APIs)
Please provide:
- CEP Number
- CEP Issue Date
- CEP Expiry Date
- CEP Certificate with all annexes
References:
Appendix 6 : Guideline On Regulatory Control Of Active Pharmaceutical Ingredients (APIs)
Only applicable for CEP option
The PRH should submit a copy of the most current CEP including all annexes, together with the following:
- A written assurance that no significant changes in the manufacturing methods or processing have taken place following the granting of the certificate or its last revision and
- A declaration from the API Manufacturer that the PRH and the NPCB shall be notified of any future change in the API specifications or in the manufacturing process that will likely affect the product’s quality or safety.
Note: All such written statements must state the name of the finished product (product name, dosage form and product strength) to be registered and the PRH shall responsible for finished product registration.
The Quality Overall Summary (QOS) is a summary that follows the scope and the outline of the Body of Data in Module 3. The QOS should not include information, data or justification that was not already included in Module 3 or in other parts of the CTD. The QOS should include sufficient information from each section to provide the Quality reviewer with an overview of Module 3. The QOS should also emphasize critical key parameters of the product and provide, for instance, justification in cases where guidelines were not followed. The QOS should include a discussion of key issues that integrates information from sections in the Quality Module and supporting information from other Modules (e.g. qualification of impurities via toxicological studies discussed under the CTD-S module), including cross-referencing to volume and page number in other Modules. This QOS normally should not exceed 40 pages of text, excluding tables and figures.
Reference ICH Guidelines:
- The Common Technical Document For The Registration Of Pharmaceuticals For Human Use: Quality – M4Q(R1)
Attachment of API Submission Checklist (Summary of Required Documents for API Information).
- International non proprietary names / INN
- Chemical names
- Synonyms
- CAS No
- Structural formula (relative and absolute chemistry)
- Molecular formula
- Molecular weight
- Molecular weight (base)
The PRH is responsible to confirm the availability of the following information:
- Physical form
- Solubility
- Melting point
- Hygroscopicity
- Polymorphism
- Particle size
- Isomerism
- Chirality
- pKa
- Partition coefficient (log P)
- pH
- Others
Manufacturers involved in each production steps, including intermediate manufacturer, milling and micronization sites.
* GMP certificate/evidence is required for all manufacturer involved in API manufacturing process, including intermediate manufacturing, milling and micronization sites
*Please refer template Declaration on Good Manufacturing Practice (GMP) Compliance for Active Pharmaceutical Ingredient (API) Intermediate Manufacturer by Competent Person
- Name and address of manufacturer that produced the API
- Attach GMP certificate in S9
- Please ensure name and address including state and postal code are the same as stated in DMF/ACTD/CEP and/or GMP certificate.
- Please provide attachment for S2.1 Manufacturers detail under S10.
State the name of synthesis route.
(If no specific name was assigned, please state as “Only One Route”).ACTD option:
- Detailed Description of the Synthesis (step & process) from starting materials until purification step.
- Proposed starting material.
- Manufacturing scheme that indicates molecular formula; molecular weights; chemical structures of starting materials, intermediates and the API including stereochemistry; reagents, catalysts and solvents used in each step until purification step.
- Catalyst & solvents used (ICH class & limit).
- Control strategy of solvents. (if skip testing, etc).
- Quantities of materials used, operating conditions and yield ranges in the description of the process.
- Recycling of filtrates/mother liquors (maximum holding time /maximum number of times the material may be recycled/Evidence / Data on the impurity levels).
- Final Steps (eg. Purification procedure).
- Commercial and Maximum batch size (batch range in kg).
- Alternatives steps (no changes in the impurity profile).
- Re-processing; identified the process/step, method, frequency/limit of reprocess, same specification of the final API, no changes in the impurity profile, controlofimpuritylevels, etc.
- Reworking: equivalent quality as original process, impurity profile, etc
- Recovery of materials or solvents: step its introduced in the process, source, ratio (or range of mixtures) of fresh and recovered solvents, specifications (including justification of specification), impurity levels.
- Blending of batches; each batch tested & comply to final API specification.
DMF option:
Closed part of the DMF: S2.2 Manufacturing Process and Process Control
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP option:
Optional
ACTD option:
Manufacturing Process Flow that indicates molecular formula; molecular weights; chemical structures of starting materials, intermediates and the final API, including its stereochemistry; reagents, catalysts and solvents used in each step until purification step.
DMF option:
Closed part of the DMF: S2.2.1 Manufacturing Process Flowchart
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP option:
Optional
ACTD option:
- i) Starting materials; Justification on selection of starting materials, Specification, Name & address of each supplier, CoA of starting material issued by each of suppliers, CoA of starting material issued by the API manufacturer (for each of suppliers), Preparation of starting materials (Brief description), characterisation.
- ii) All materials (solvent, catalyst or reagent) used during manufacturing process [Specification, function and control strategy].
iii) Others. e.g. benzene contamination, Quality of water etc.
S2.3.1a TSE Risk Free Statement
- Declaration; starting materials, reagents and all materials used to manufacture the API are of animal or human origin.
- ii) Document to demonstrate compliance on TSE/BSE requirement
DMF option:
Closed part of the DMF: S2.3Control of Materials
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP option:
Optional
ACTD option:
Controls of Critical Steps
- critical steps & process control including tests and acceptance criteria (with justification including experimental data).
Controls of Intermediates
- List of Intermediates, specification, analytical procedure
DMF option:
Closed part of the DMF: S2.4 Controls of Critical Steps & Intermediates
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP option:
Optional
ACTD option:
Applicable to sterile API only.
DMF option:
Closed part of the DMF: S2.5 Process Validation and/or Evaluation.
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP Option:
Required for sterile API if CEP did not specify API as Sterile Grade.
ACTD option:
- Description and discussion of significant changes made to the manufacturing process and/or manufacturing site of the API used in producing non-clinical, clinical, scale-up, pilot and if available, production scale batches.
- The development history of the manufacturing process as described in S 2.2
- To state the date of changes.
DMF option:
Closed part of the DMF: S2.6 Manufacturing Process Development.
*For DMF option, the complete DMF (open & closed part) should be submitted via electronic copy (CD) directly to the NPRA to maintain confidentiality of the contents.
CEP option:
Optional
- Description & characteristics of various polymorphic forms
- Potential for formation of the polymorphic forms
- Stability of the polymorphic forms
- Evidence to prove the commercial scale process consistently produce desired polymorphic forms
2. Particle size distribution.
3. Isomerism- Discussion on isomerism including configuration of chiral centers, geometric isomerism cis/trans or E/Z (if isomer exists).
4. Discussion on studies of elucidation of structure and other characteristics.
Pharmacopoeial API:
Comparison of spectral data between pharmacopoeial reference standard & API (If comparison is not available, assess as per nonpharmacopoeial API).
Non Pharmacopoeial API:
- Elemental analysis
- Infrared Spectrophotometry (IR)
- Ultraviolet absorption spectrum (UV)
- Mass spectrometry
- Nuclear Magnetic Resonance Spectrometry (NMR)
- X-ray Diffraction
- Differential Scanning Calorimetry (DSC)
- Thermogravimetric analysis (TGA)
- Others
- Discussion of the possible carryover of all impurities (organic impurities (actual and potential impurities), residual solvents, inorganic impurities and genotoxic impurities) that may arise during the synthesis of API, from the preparation of starting material and intermediate to the final API.
- Discussion on impurities with regard to impurities with potential genotoxicity. If, on the basis of the discussion, a genotoxic impurity is indeed liable to be present in the substance, then please demonstrate that it comply with the current genotoxic guidelines.
- Discussion on the potential impurities that may arise from the starting materials, route of synthesis and possible degradation products should be listed with name, structure, origin, LOD, LOQ and range of results in at least 3 consecutive batches as well as the proposed limits taking into account the requirements of ICH guideline.
- Any impurity greater than qualification threshold should be qualified and a rationale for establishing impurity limit/ acceptance criteria that includes safety considerations (eg. data from toxicology study, or batch analysis data of batches used in clinical trial with observed impurites content are equal or more than limit in the specification) should be provided.
- Discussion on impurities that stated in other pharmacopeia (if applicable)
- Impurities:
- Specified, Unspecified, Total impurities, Residual solvents, Inorganic
Specification of API (with specification version no. & effective date):
- From API Manufacture (*in-house/pharmacopoeial)
- From Finished Product Manufacturer (*in-house/pharmacopoeial)
Applicable to DMF & ACTD options:
The PRH is responsible to ensure the availability of the following information from both API Manufacturer and Product Manufacturer:
- Calculation or equation used in the protocol.
- Standard Testing Procedure for each specification
Applicable to DMF & ACTD options
The PRH is responsible to ensure the availability of the following information from API Manufacturer:
- Partial validation analytical procedure and report together with chromatograms raw data (accuracy, precision, specificity, intermediate precision, LOO, LOQ, linearity, range and system suitability testing).
- Validation data on organic impurities
- The test method used other than those described in the reference shall be fully described and validated.
- Batch analysis results of at least 3 batches from API manufacturer
- Information in table form
e.g.: batch number, batch size, manufacturing date, manufacturing site and batch use (validation, stability, commercial etc.)
*From API manufacturer AND finished product manufacturer
- Provide justification of specification for all test parameters & limits in final API specification.
- Discussion on inclusion/ omission of tests and analytical procedures
Information from API Manufacturer & Product Manufacturer are required:
- Discussion on inclusion/ omission of tests and analytical procedures
- Justification on range of acceptance criteria set for in-house tests
Please provide from both API manufacturer and finished product manufacturer
State the details of reference standard:
- Official reference standard used (with batch number)
- Primary reference standard used (with batch number)
- Working standard used (with batch number)
Provide attachment:
- CoA of primary references standard
- CoA of working standard (working standard should be qualified against official/primary reference standard)
- Individual IR spectra of primary and working standards
- Overlaid IR spectra comparing the official/primary and working standards
- Reference standards available for impurities/related substances
- If the submission option choose is CEP, please provide justification & discussion on using other reference standard instead of Ph. Eur. reference standard.
- State clearly the container closure system used for storage of the API
- Identity of the material used for each component (for primary and secondary packaging)
- Specification and CoA for primary packaging material
- IR spectra of the primary packaging material
- Functional secondary packaging components.
- A discussion on suitability (eg: choice of materials, protection from moisture and light, compatibility of the materials of construction with the API, and/or safety of materials of construction).
State the proposed retest period based on stability data provided.
State the proposed shelf life based on stability data provided.
Stress testing data:
- Provide data for stress testing study (state batch no., stress condition and testing parameter etc.) (E.g. moisture, light, acidic, basic, oxidative and thermal stress conditions)
Long term stability data:
- Provide batch size, batch number and manufacturing date for each batches mentioned in the study.
- Clarify on packaging material used during stability study.
- Provide current long term stability data (at least 3 commercial batches or 3 pilot batches) to support the proposed retest period.
- For finished product manufactured at Zon IVB, long-term stability data conducted at Zon IVB, 30 oC ± 2 oC/75% RH ± 5% RH (if available) should be provided.Otherwise, written stability commitment that long-term stability will be conducted at Zon IVB, 30 oC ± 2 oC/75% RH ± 5% RH should be provided.
Accelerated stability data:
- Provide batch size, batch number and manufacturing date for each batches mentioned in the study.
- Clarify on packaging material used during stability study.
- Provide current accelerated stability data (at least 3 commercial batches or 3 pilot batches) to support the proposed retest period.
Post-approval Stability Protocol and Stability Commitment
- Provide stability study commitment
State the proposed API storage condition (including special label, if needed) based on study condition of stability data provided (eg: Store below 25oC, protect from light).
- Attachment of DMF open part (optional)
- DMF Version Number: Current version number with date
Note: The API Manufacturer should submit the DMF via electronic copy (CD) directly to Centre of Product Registration of NPRA to maintain confidentiality of the contents. The information contained in the closed part of the DMF will be regarded as confidential and will only be evaluated in support of the applications mentioned in the Letter of Access. The confidential information will not be disclosed to any third party without a written authorization from the API Manufacturer. The PRH is responsible to ensure that the complete DMF (i.e. both the Open part and the Closed part) submitted to NPRA directly by the API Manufacturer.
The DMF should reach NPRA at the point of screening submission. Failure to do so may result in submission rejection.
Applicable to DMF option ONLY
The Letter of Access from API Manufacturer/ holder of the DMF authorizes the NPRA to refer to the DMF, in support of the application for a finished product. Thus, the Letter of Access must state the following:
- The name of the finished product (product name, dosage form and product strength) to be registered;
- The PRH responsible for finished product registration; and,
- Adeclaration that both the PRH and the NPRA shall be notified of any change in the API specification or in the manufacturing process that will likely affect the product’s quality or safety.
Applicable to DMF & ACTD option
- Provide valid GMP certificate for API manufacturer declared in S2.1
- Select GMP issuing body
- Date of issue of GMP certificate
- Date of expiry of GMP certificate







