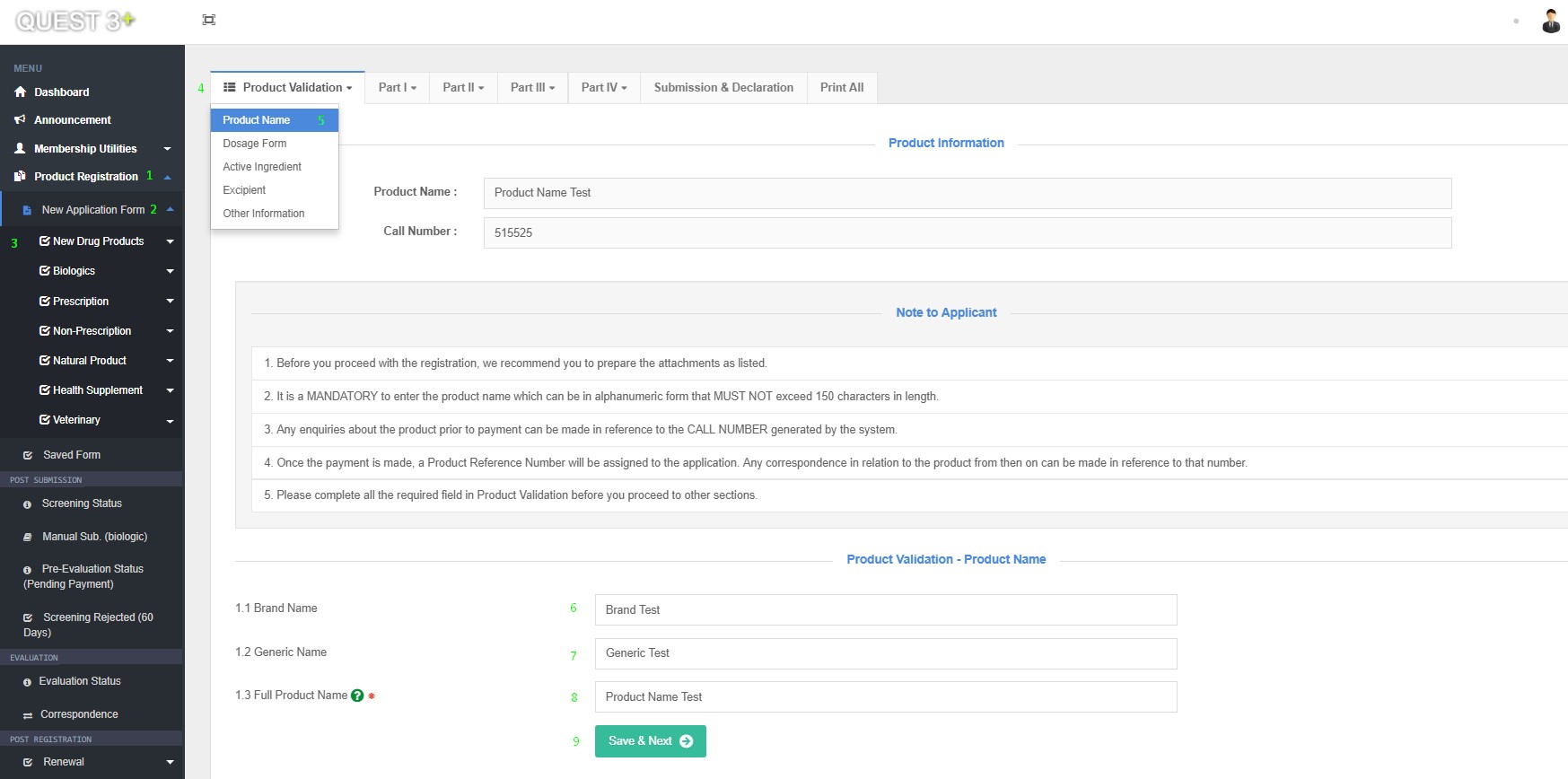
Product Name
1.1 Brand Name
1.2 Generic Name
1.3 Full Product Name
Product name is defined as a name given to a product which may either be a proprietary name (an invented name); or a generic name (common name) or scientific name, together with a trade mark or the name of the manufacturer.
- Product name shall consist of dosage form and strength (for single active ingredient product). (e.g.XYZ Capsule 500mg)
- Any product name which is the same or similar either in writing/ pronunciation, with the product name of an adulterated product is prohibited.
- Product name may be included together with the brand name or trademark name, if applicable.
- Please check product names which are not permitted to be registered as specified in DRGD.
Reference National Pharmacy Regulatory Agency (formally known as National Pharmaceutical Control Bureau):
Drug Registration Guidance Document (DRGD) – Appendix 9 under 9.1.4 Table 2 List of Non-Permissible Product Names.
Traditional Medicines:
- To use a formulary name, any amendments made to the product formulation such as the addition of active ingredients, removal of active ingredients or change in strength of active ingredients will not be permitted.
- A brand name in front of the formulary name shall be required to be added, in order to differentiate and identify that their product from products with the same formulary name.
- For products in which the product name is the name of active ingredient or the product name is a common name, e.g. Kapsul Kacip Fatimah; Misal Kucing Tea; Ortosiphon Capsule; Herbal Rub; Natural Herb Capsule, a brand name shall be added to the product name, in order to differentiate and identify this specific product.
- Justification will be required to prove the “claim” made in the product name. Example: “Double Strength/ Acticoat/WaterSol”
- Product name supported by a registered trade mark certificate will not be accepted if deemed inappropriate by the Authority/ not following the regulations stated in this appendix.
- Replacement product may use the same product name as a previously registered provided that the formulation (strength of active ingredient), product registration holder and dosage form of the product remains the same.
- The name of the active ingredient is not allowed to be used as brand name.
- The name of active ingredient combined with product indication is not allowed to be used as product name.
Reference National Pharmacy Regulatory Agency (formally known as National Pharmaceutical Control Bureau):
Drug Registration Guidance Document (DRGD) - Appendix 5 under 2.4 Table 7 List of Non-Permissible Product Name for Traditional Medicines.
How to Access Product Name in QUEST System ?
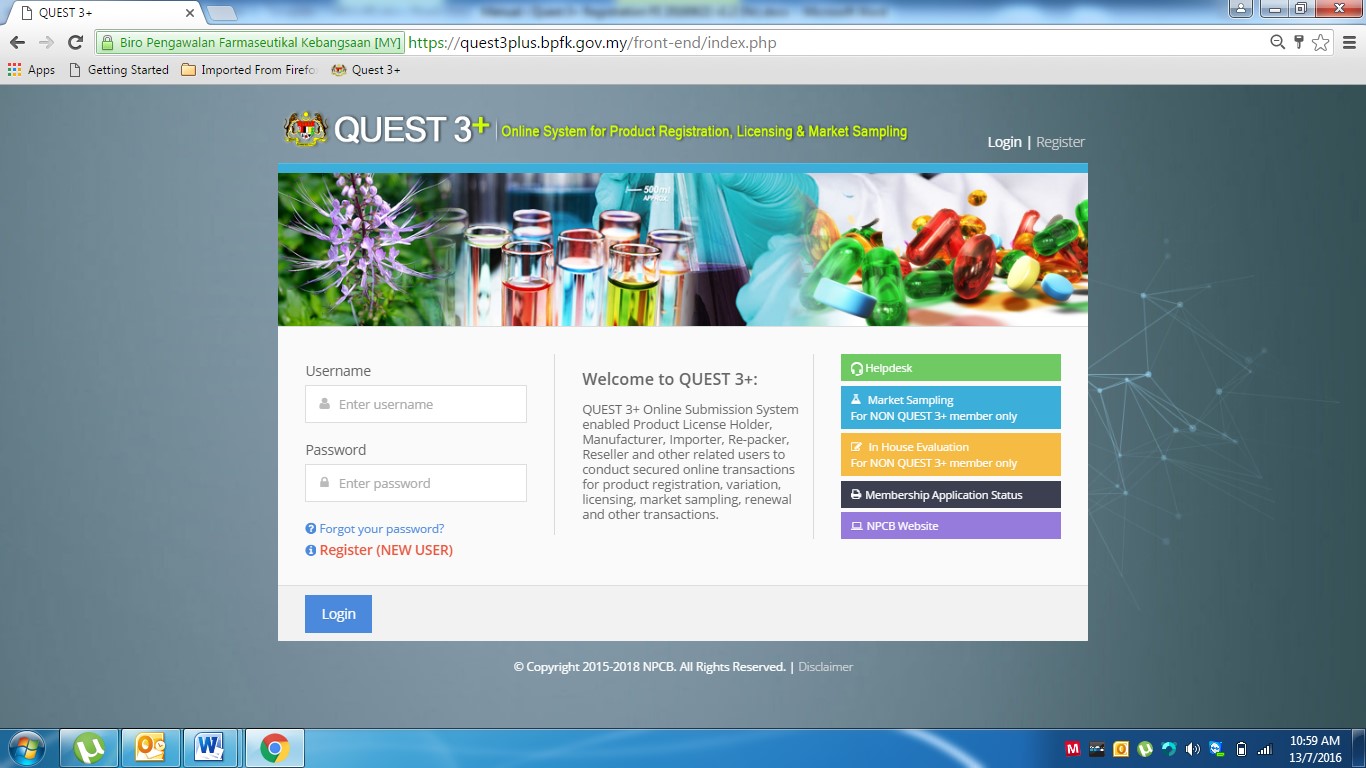
To access the Quest 3+ system, user need to use the URL as below:
https://quest3plus.bpfk.gov.my/front-end
Figure 1 as the above will appear and the user will need to enter the following information:
1. User Name: User ID
2. Password: Enter Password.
3. Click Login
After successful log in, Submission form can be access here : Product Registration >> New Application Form >> Choose Product Category >> Product Validation >> Product Name
Fill in this Fields and click 'Save & Next' button :
1.1 Brand Name
1.2 Generic Name
1.3 Full Product Name