MADRAC Bulletin
The MADRAC Bulletin also features pharmacovigilance-related activities conducted by the National Pharmaceutical Regulatory Agency (NPRA) and contains a list of directives based on safety issues advised by the Malaysian Adverse Drug Reactions Advisory Committee (MADRAC) and endorsed by the Drug Control Authority (DCA) as well as safety alerts that have been published on the NPRA website.
To receive the latest issues of MADRAC Bulletin, you may join the NPRA Safety Information Mailing List by completing the subscription form.
The MADRAC Bulletin is intended for healthcare professionals and is only available in English.
MADRAC Bulletin Vol. 57 Issue 06/2025
Highlights
-
10th Anniversary of #MedSafetyWeek Campaign
- Soft launch of #MedSafetyWeek 2025
Articles Based on Case Reports
-
Reports of Suicidal Behaviour with Efavirenz Use
MADRAC Bulletin Vol. 56 Issue 05/2025
Articles Based on Case Reports
-
Isotretinoin: Risk of Psychiatric Adverse Effects
Features
- Pharmacovigilance Seminar: Enhancing Patient Safety - Pharmacists at the Frontline of Product Monitoring [Training]
- Get ready for #MedSafetyWeek 2025
MADRAC Bulletin Vol. 55 Issue 04/2025
Articles Based on Case Reports
-
Tacrolimus-Induced Toxic Leukoencephalopathy
Features
- Workshop on Effective Social Media Content Creation [Training]
MADRAC Bulletin Vol. 54 Issue 03/2025
Articles Based on Case Reports
-
Asparaginase-Associated Pancreatitis (AAP) in Paediatric Patients
Features
- Early Planning for #MedSafetyWeek 2025: A United Effort for Safer Medicines [Collaboration]
- Internal Workshop Highlights: Data Mining, Data Visualisation, and Article Writing [Training]
MADRAC Bulletin Vol. 53 Issue 02/2025
Highlights
-
Understanding Immunisation Stress-Related Responses (ISRRs)
Articles Based on Case Reports
-
Sleepwalking with Zolpidem
Features
- Welcome MADRAC 2025-2027
MADRAC Bulletin Vol. 52 Issue 01/2025
Articles Based on Case Reports
-
Dacomitinib: Local Case Reports on Palmar-Plantar Erythrodysesthesia Syndrome (PPES)
-
Dupilumab-Induced Arthralgia
Features
-
Adverse Event Reports Received 2015-2024
MADRAC Bulletin Vol. 51 Issue 06/2024
Signals
-
Amlodipine: A Reminder on the Risk of Gingival Enlargement
Features
-
Pharmacovigilance in the Pharmaceutical Industry: Latest updates from NPRA [Training]
-
#MedSafetyWeek 2024: The Malaysian story in pictures
-
Thank You MADRAC 2022-2024
MADRAC Bulletin Vol. 50 Issue 05/2024
Highlights
- Research@NPRA
- Strategies to Enhance Risk Communication About Medicines in Malaysia: A Delphi Study Among Multinational Experts
Articles Based on Case Reports
- Azathioprine: Drug-induced Liver Injury (DILI)
- Wooden Chest Syndrome: Chest Wall Rigidity with Fentanyl
MADRAC Bulletin Vol. 49 Issue 04/2024
Signals
-
Ribociclib and Blood Creatinine Increased
Features
-
[New!] Infographic of Paracetamol: Reports of Fixed Drug Eruptions
Articles Based on Case Reports
-
Methotrexate-Induced Posterior Reversible Encephalopathy Syndrome (PRES) in a Paediatric Patient
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 48 Issue 03/2024
Highlights
-
Research@NPRA
- Using Interpretable Machine Learning to Identify Factors That Influence the Quality of Malaysian Adverse Event Reports
Articles Based on Case Reports
-
Allopurinol-Induced Photosensitivity Reaction
-
Allopurinol Reminder Infographic
-
Features
- Training
- Pharmacovigilance Seminar: Monitoring of Registered and Special Approval Medicines
- Uganda National Drug Authority Attachment at NPRA
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 47 Issue 02/2024
Articles Based on Case Reports
-
Clomiphene: Risk of Serious Visual Disturbances
-
Domperidone: Risk of Seizures
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 46 Issue 01/2024
Signals
- Atorvastatin and Photosensitivity Reaction
Articles Based on Case Reports
- Clozapine-Induced Myocarditis
Features
- Adverse Event Reports Received 2014-2023
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 45 Issue 06/2023
Articles Based on Case Reports
- Watch Out for PRIS! - A Local Case Report on Propofol-Related Infusion Syndrome
- When the Liver Shouts S.O.S. - A Case Report on Oxaliplatin-Induced Sinusoidal Obstruction Syndrome (SOS)
Features
- Training
- Pharmacovigilance: Our Shared Responsibility - A Seminar for Doctors, Pharmacists and Nurses
- Publication
- Thrombocytopenia and Venous Thromboembolic Events after BNT162b2, CoronaVac, ChAdOx1 Vaccines and SARS-CoV-2 Infection: A Self- Controlled Case Series Study
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 44 Issue 05/2023
Signals
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome Associated with Antiretroviral Therapy (ART)
- Efavirenz
- Tenofovir/Emtricitabine
Features
- Publication
- Carbamazepine-Induced Severe Cutaneous Adverse Drug Reactions: A 21-Year Comparison Between Children and Adults in Malaysia
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 43 Issue 04/2023
Signals
- Drug-induced Nephrotoxicity
- Colistin (Polymyxin E) – Acute Kidney Injury (AKI)
- Imatinib – Renal disorders
Features
- Publication
- Hearing Loss and Tinnitus Associated with COVID-19 Vaccines:
An Analysis from the National Pharmacovigilance Database in Malaysia
- Hearing Loss and Tinnitus Associated with COVID-19 Vaccines:
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 42 Issue 03/2023
Articles Based on Case Reports
- Sodium Valproate: Risk of Acute Pancreatitis
- Risk of Acute Kidney Injury with Dabigatran in Patients with Atrial Fibrillation
Features
-
Pharmacovigilance Seminar: Pharmacovigilance for Safer Use of Medicines
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 41 Issue 02/2023
Articles Based on Case Reports
- Tenofovir Disoproxil Fumarate-Induced Fanconi Syndrome in a Patient with Hepatitis B
- Reminder on the Risk of Diplopia with High Dose and Prolonged Use of Carbamazepine
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin Vol. 40 Issue 01/2023
Highlights
- Venturing into Quantitative Signal Detection
Signals
- Cephalosporins – Severe Cutaneous Adverse Reactions (SCARs)
- Cefazolin – Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
- Cefepime – Acute Generalised Exanthematous Pustulosis (AGEP)
Features
- Adverse Event Reports Received 2013-2022
What's New?
- List of Safety Alerts/Directives Related to Drug Safety Issues
MADRAC Bulletin - 03/2022 Edition
Features
▪ Pharmacovigilance Seminar: Pharmacovigilance for Safer Use of Medicines
Articles Based on Case Reports
▪ Risk of Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT) following Vaxzevria Vaccination▪ Sacubitril/Valsartan: Risk of Psychiatric Events
▪ List of Directives Related to Drug Safety Issues
MADRAC Bulletin - 02/2022 Edition
Features
▪ Malaysian NPRA-Australian TGA Seminar on Safety Signal Management
▪ NPRA’s Training Workshops on COVID-19 Vaccine AEFI Reporting, Case Investigation and Risk Communication
▪ Summary Report on Adverse Events Following Immunisation of COVID-19 Vaccines in Malaysia #4
Articles Based on Case Reports
▪ Iron Dextran: Risk of Seizures▪ Hydroxyurea for Myeloproliferative Disorder: Risk of Cutaneous Vasculitis and Gangrene
▪ Venetoclax-Induced Tumour Lysis Syndrome
▪ Atypical Antipsychotics: Risk of Urinary Retention
▪ List of Directives Related to Drug Safety Issues
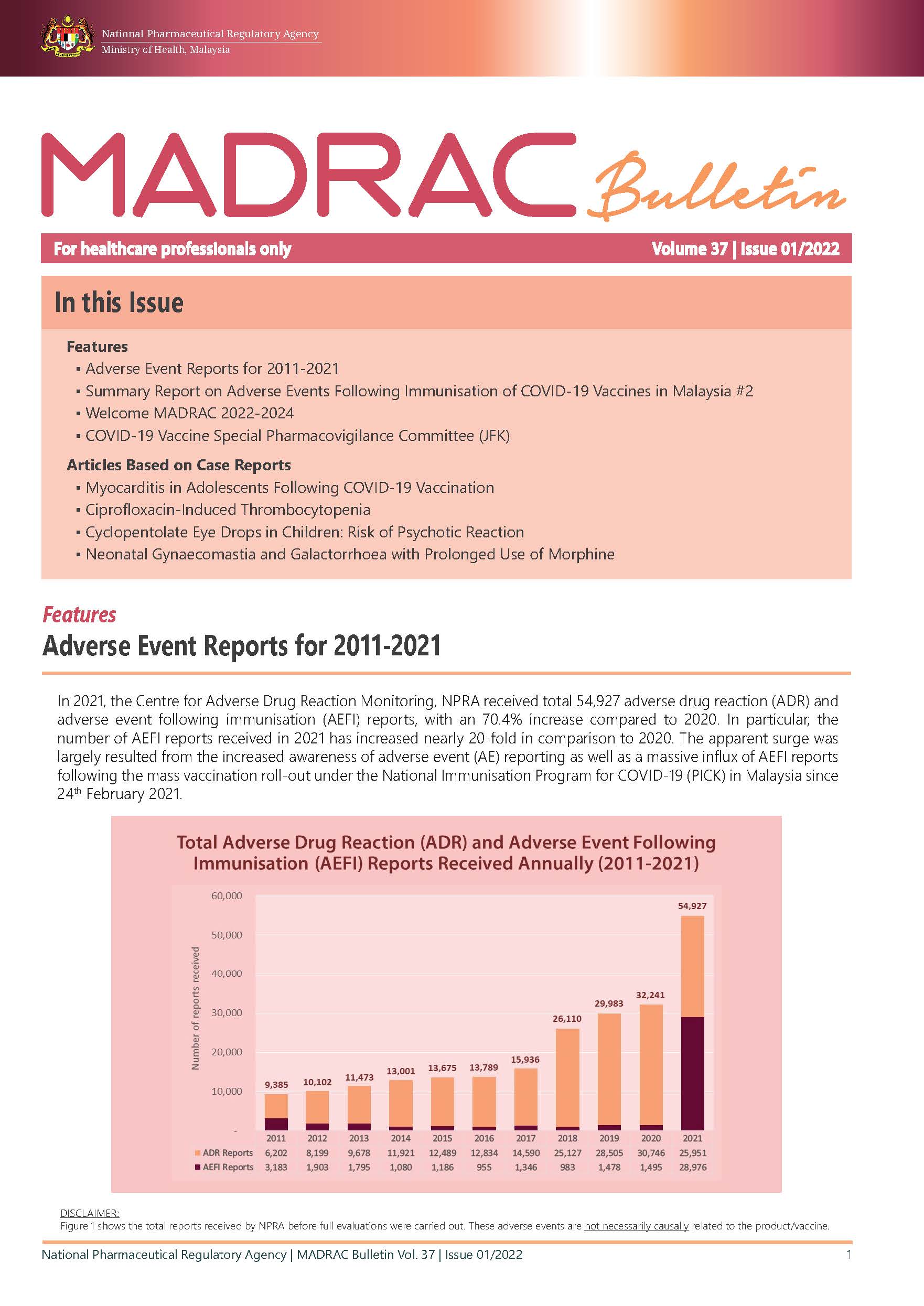
MADRAC Bulletin - 01/2022 Edition
Features
▪ Adverse Event Reports for 2011-2021
▪ Summary Report on Adverse Events Following Immunisation of COVID-19 Vaccines in Malaysia #2
▪ Welcome MADRAC 2022-2024
▪ COVID-19 Vaccine Special Pharmacovigilance Committee (JFK)
Articles Based on Case Reports
▪ Myocarditis in Adolescents Following COVID-19 Vaccination▪ Ciprofloxacin-Induced Thrombocytopenia
▪ Cyclopentolate Eye Drops in Children: Risk of Psychotic Reaction
▪ Neonatal Gynaecomastia and Galactorrhoea with Prolonged Use of Morphine
MADRAC Bulletin - 03/2021 Edition
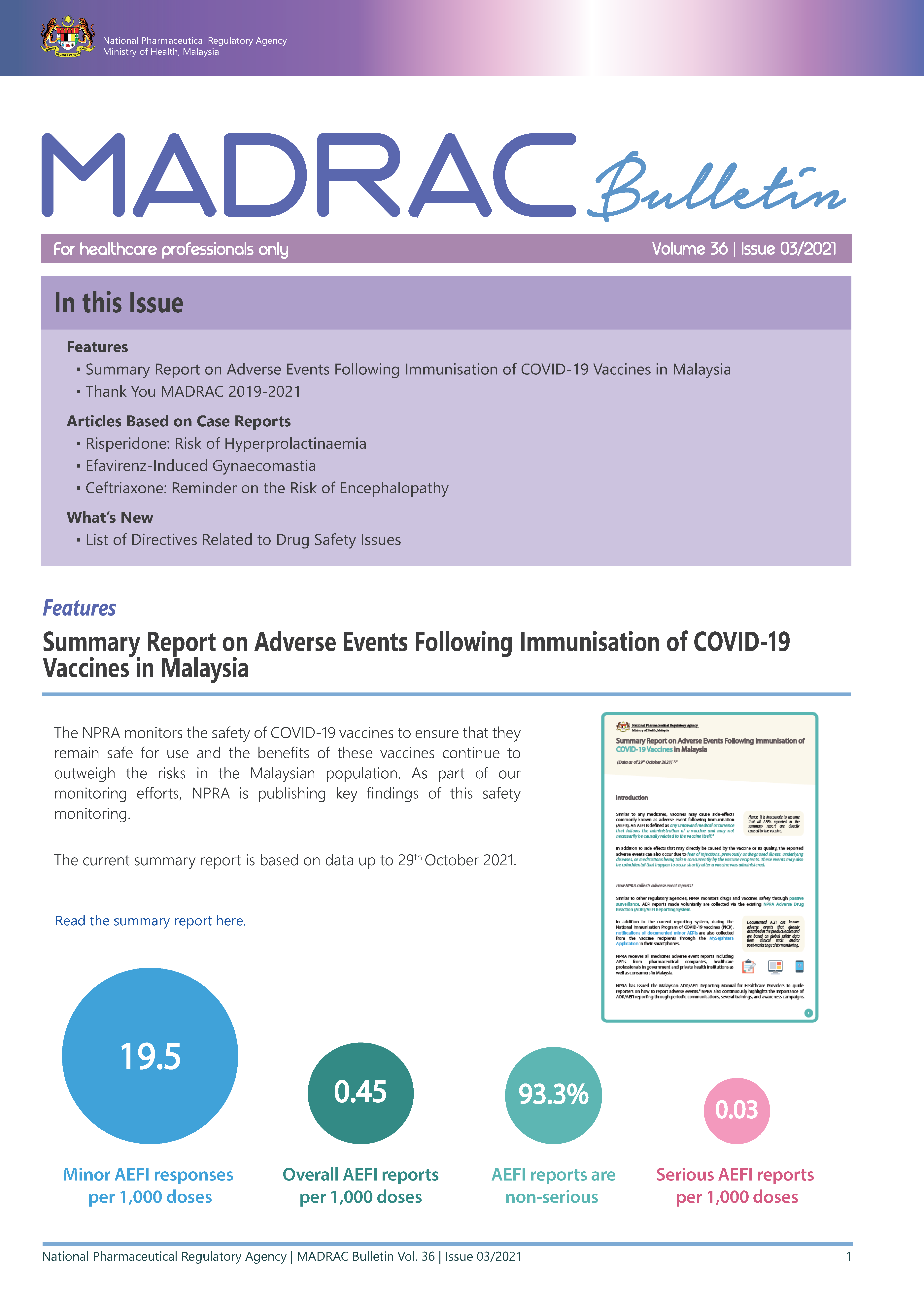
▪ Summary Report on Adverse Events Following Immunisation of COVID-19 Vaccines in Malaysia
▪ Thank You MADRAC 2019-2021
Articles Based on Case Reports
▪ Risperidone: Risk of Hyperprolactinaemia▪ Efavirenz-Induced Gynaecomastia
▪ Ceftriaxone: Reminder on the Risk of Encephalopathy
What's New?▪ List of Directives Related to Drug Safety Issues
MADRAC Bulletin - 02/2021 Edition
Articles based on Case Reports
▪ Clozapine-induced gastrointestinal hypomotility (CIGH)
▪ Dapsone-induced methaemoglobinaemia
▪ Gastrointestinal adverse effects associated with immune checkpoint inhibitors
▪ Ophthalmic preparations of vascular endothelial growth factor (VEGF) inhibitors: Risk of myocardial infarction
What’s New
▪ List of directives related to drug safety issues
MADRAC Bulletin - 01/2021 Edition
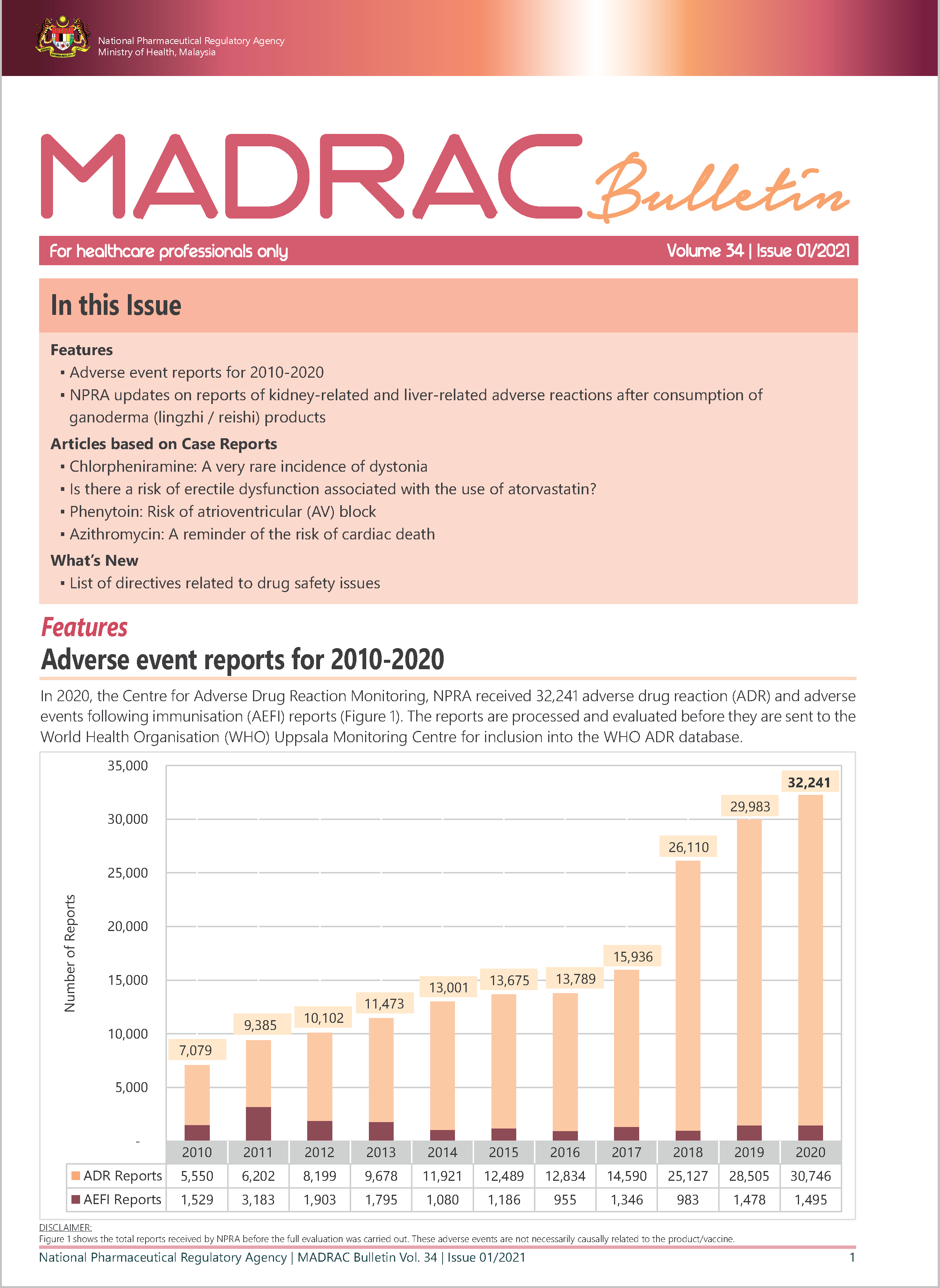
▪ Adverse event reports for 2010-2020
▪ NPRA updates on reports of kidney-related and liver-related adverse reactions after consumption of ganoderma (lingzhi / reishi) products
Articles based on Case Reports
▪ Chlorpheniramine: A very rare incidence of dystonia▪ Is there a risk of erectile dysfunction associated with the use of atorvastatin?
▪ Phenytoin: Risk of atrioventricular (AV) block
▪ Azithromycin: A reminder of the risk of cardiac death
What's New?▪ List of directives related to drug safety issues
MADRAC Bulletin - 03/2020 Edition
▪ Shoulder injury related to vaccine administration (SIRVA): A preventable occurrence
▪ Jarisch-Herxheimer reaction: An adverse drug reaction following treatment with benzathine penicillin in syphilis patients
▪ Rapid correction of hyponatraemia with intravenous saline solution causing osmotic demyelination syndrome
▪ Sulfamethoxazole-trimethoprim-induced drug reaction with eosinophilia and systemic symptoms (DRESS)
MADRAC Bulletin - 02/2020 Edition
▪ Preliminary report: Local adverse drug reactions associated with drugs used for COVID-19 infections
Articles based on Case Reports
▪ Hydrochlorothiazide: Risk of hearing disorder▪ Amlodipine and peripheral neuropathy
▪ Ceftazidime: Risk of acute generalised exanthematous pustulosis (AGEP)
What's New?▪ List of Directives Related to Drug Safety Issues (May - August 2020)
MADRAC Bulletin - 01/2020 Edition
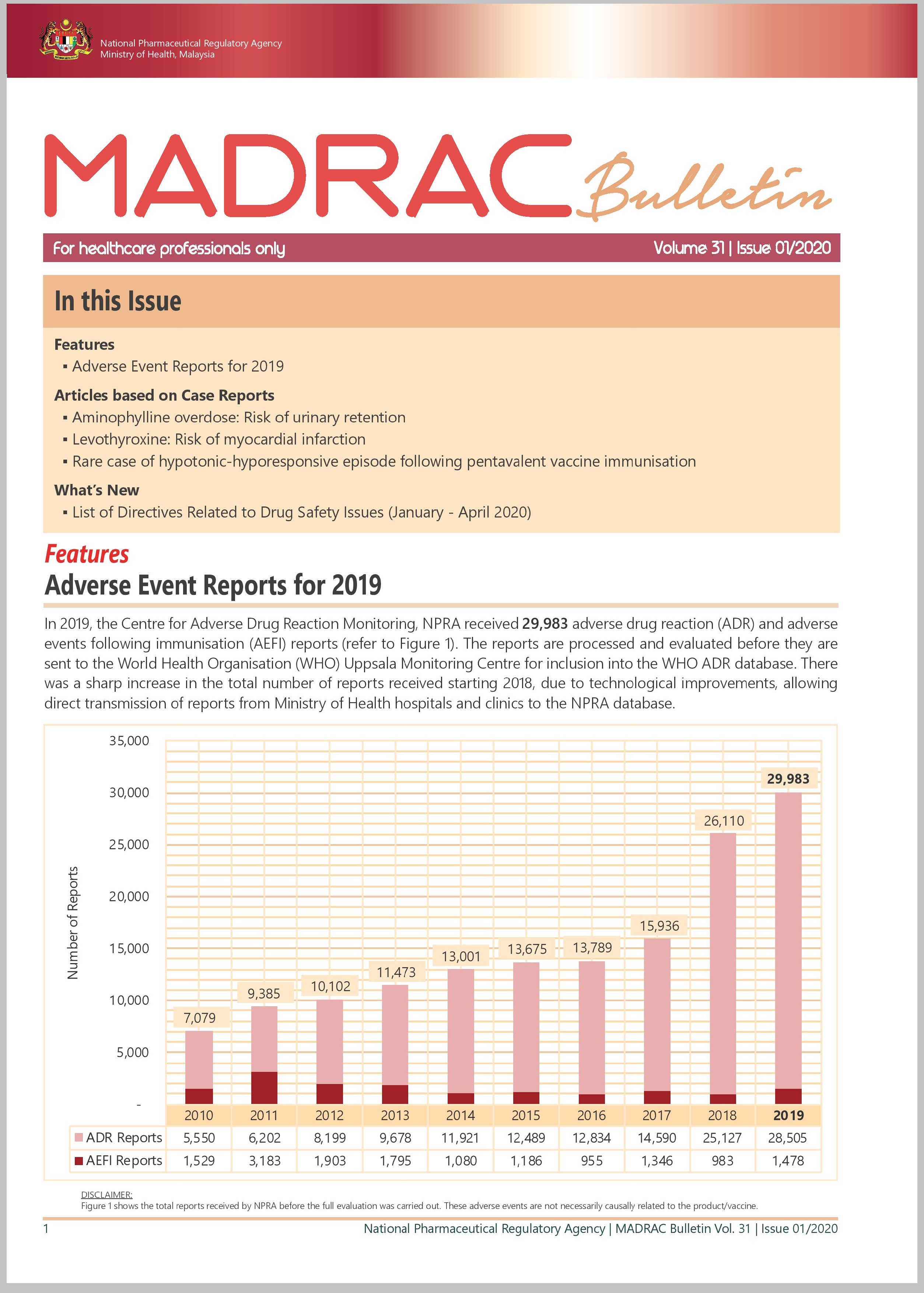
▪ Adverse Event Reports for 2019
Articles based on Case Reports
▪ Aminophylline overdose: Risk of urinary retention
▪ Levothyroxine: Risk of myocardial infarction
▪ Rare case of hypotonic-hyporesponsive episode following pentavalent vaccine immunisation
What's New?
▪ List of Directives Related to Drug Safety Issues (January - April 2020)
MADRAC Bulletin - 03/2019 Edition
▪ Erythropoiesis-stimulating agents: A reminder on the cardiovascular risk in patients with chronic kidney disease
▪ Amiodarone: Risk of acute hepatitis
▪ Levothyroxine: Reminder on the risk of restlessness or irritability
▪ Immunisation anxiety-related reactions
What's New?
▪ List of Directives Related to Drug Safety Issues (September - December 2019)
MADRAC Bulletin - 02/2019 Edition
▪ NPRA's ADR Workshop for Pharmacists
Articles based on Case Reports
▪ Zidovudine: Higher Occurrence in Malaysia?
▪ Colchicine: Reminder on the Risk of Rhabdomyolysis
▪ Pembrolizumab: Reminder on the Risk of Pneumonitis
What's New?
▪ List of Directives Related to Drug Safety Issues (May - August 2019)
MADRAC Bulletin - 01/2019 Edition
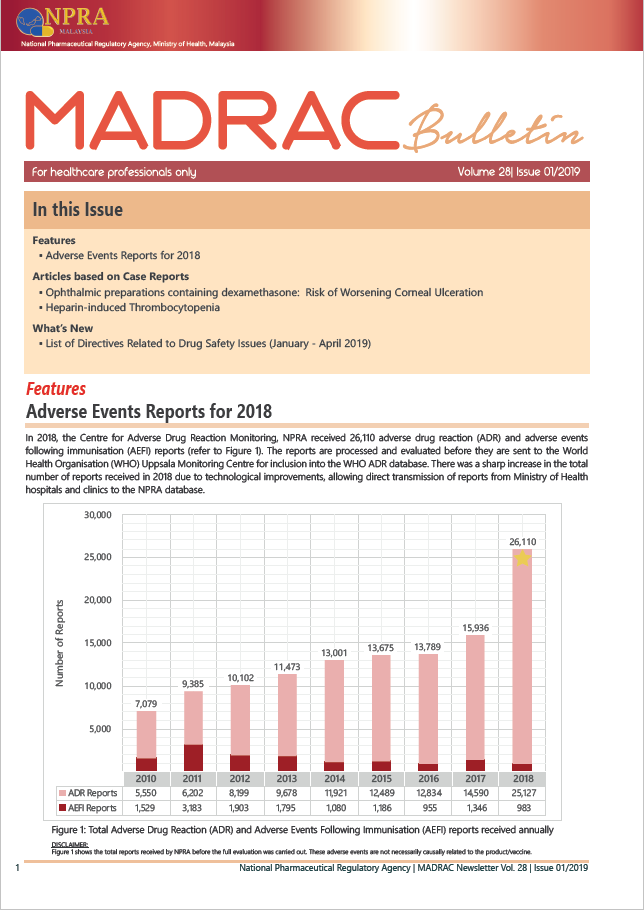
▪ Adverse Event Reports for 2018
Articles based on Case Reports
▪ Ophthalmic preparations containing dexamethasone: Risk of Worsening Corneal Ulceration
▪ Heparin-induced Thrombocytopenia
What's New?
▪ List of Directives Related to Drug Safety Issues (January - April 2019)
MADRAC Bulletin - 03/2018 Edition
▪ Thank You MADRAC 2016-2018
Articles based on Case Reports
▪ Amlodipine: Reminder on the Risk of Increase in Urinary Frequency and Nocturia
▪ SGLT-2 Inhibitors: Local Reports of Euglycaemic Diabetic Ketoacidosis
▪ Atezolizumab: Reminder on the Risk of Hyperthyroidism
What's New?
▪ List of Directives Related to Drug Safety Issues (September - December 2018)
MADRAC Bulletin - 02/2018 Edition
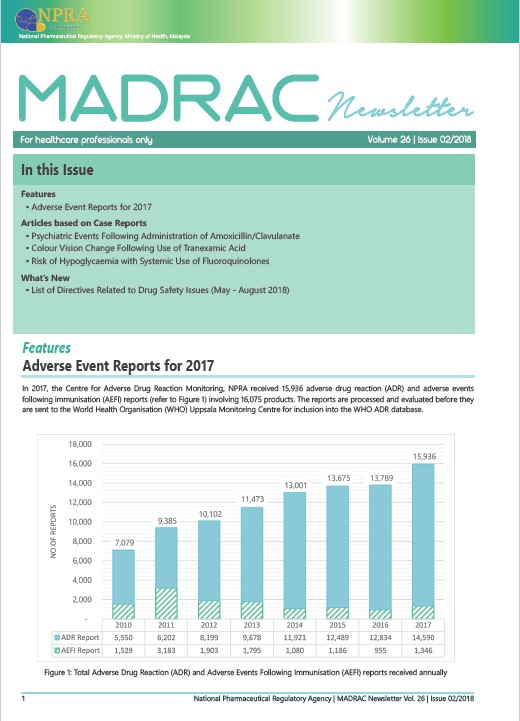
▪ Adverse Reaction Report for Year 2017
Articles based on Case Reports
▪ Psychiatric Events Following Administration of Amoxicillin/Clavulanate
▪ Colour Vision Change Following Use of Tranexamic Acid
▪ Risk of Hypoglycaemia with Systemic Use of Fluoroquinolones
What's New?
▪ List of Directives Related to Drug Safety Issues (May - August 2018)
MADRAC Bulletin - 01/2018 Edition
Features
▪ Recent Safety Issues with Fluoroquinolones
Articles based on Case Reports
▪ Simvastatin: Reminder on the Risk of Cognitive Impairment
▪ Dipeptidyl Peptidase-4 Inhibitors: Reminder on the Risk of Pancreatitis
▪ Povidone-iodine Gargle: Risk of Hypothyroidism
▪ Adulteration of Traditional Products with Corticosteroids: Risk of Psychiatric Disorders
What's New?
▪ List of Directives Related to Drug Safety Issues (January - April 2018)
|
MADRAC Bulletin - 03/2017 Edition Safety Issues Presented to MADRAC in 2017 Regulatory Matters ADR Reporting
|
|
MADRAC Bulletin - 02/2017 Edition Regulatory Matters
|
|
MADRAC Bulletin - 01/2017 Edition Features Regulatory Matters What’s New?
|
|
MADRAC Bulletin - Disember 2016 Edition Regulatory Matters
|
|
MADRAC Bulletin- Ogos 2016 Edition Annual Report for 2015
|
|
MADRAC Bulletin - April 2016 Edition
|
|
MADRAC Bulletin - Disember 2015 Edition
|
|
MADRAC Bulletin - Ogos 2015 Edition
|
|
MADRAC Bulletin - April 2015 Edition
|
|
MADRAC Bulletin 2014 - Disember Edition - Macrolides: Overview of Local ADR Data and Updates on the Risk of QT Interval Prolongation |
|
MADRAC Bulletin 2014 - Ogos Edition - Annual Report for 2013: |
|
MADRAC Bulletin 2014-April Edition - Individual Genetic Make-up, A Key to Personalised Medicine |
|
- Hydroxyethyl Starch (HES)-containing Products: New Safety Updates due to Recent Published Data on Increased Mortality and Risk of Kidney Injury Requiring Dialysis |
|
- Carbimazole vs. propylthiouracil (PTU): A comparison of drug safety profiles based on spontaneous ADR reports in Malaysia |
|
- Welcoming the MADRAC Committee for 2013-2015 |
|
Special 25th Anniversary Edition |
|
- sAllopurinol: Update on the usage in MoH facilities and related adverse cutaneous drug reactions
|
|
- Activities of MADRAC in 2011 |
|
- Erythropoiesis-stimulating agents (ESA) in chronic kidney disease: Modified dosing recommendations |
|
MADRAC ADR 2011 - Ogos |
|
- Activities of MADRAC in 2010 |
|
- Avandia (Rosiglitazone)& Combination Products: Restriction of Use Due to Cardiovascular Risk |
|
- Mucolytic Agents: Contraindication in Children Below 2 Years of Age |
|
- ADR Reporting for 2009: An Overview |
Newsletter: MADRAC Bulletin / Archive