Veterinary Medicine
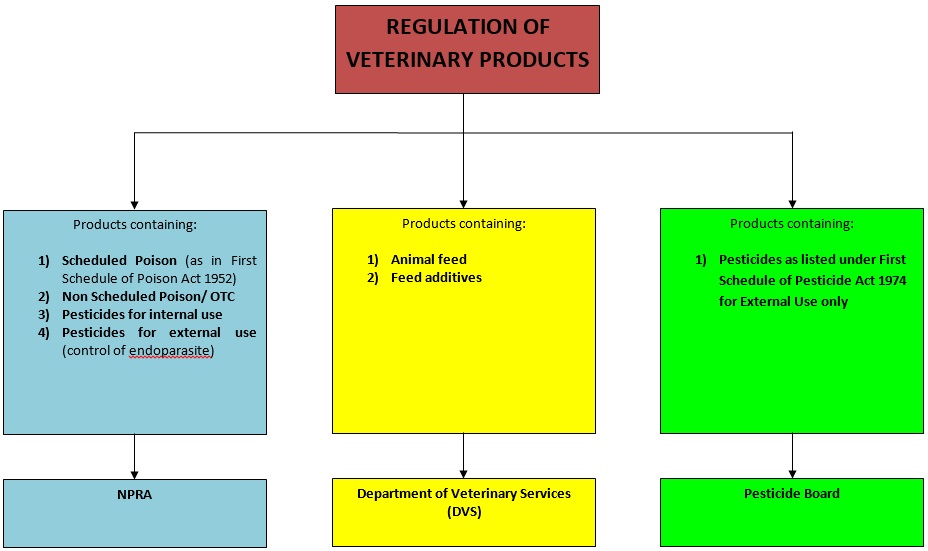
- Products containing feed additives in combination with scheduled poisons will be regulated by the DCA.
- Products containing pesticide ingredients in combination with scheduled poisons will be regulated by the DCA.
Recent Updates, Directives & Circulars for Veterinary Medicine
- Recent Updates for Guidelines, Circulars, Directives, FAQ & Announcements for Veterinary Medicine
- Directives & Circulars Related to Veterinary Medicine
List of Renewal Approved Veterinary Products |
MADRAC Members |
Ahli MADRAC |
Direktif Berkenaan Pengemaskinian Garis Panduan Malaysian Guideline For Application Of Clinical Trial Import Licence (CTIL) And Clinical Trial Exemption (CTX) |
|
|
Product Registration Process
Step I : Preparation
Product classification, Token Configuration, Payment Mode and Key Documents
Pre-submission of Application (Preparation)
Step I : PreparationStep 2 : Submission
Key-in, upload documents and submission of online application forms (Part I, Part II, Part III & Part IV)
Product Validation, Part I, Part II, Part III & Part IV
Step 2 : SubmissionStep 3 : Regulatory Outcome
Evaluation of Application & Drug Control Authority (DCA) decision.
Evaluation of Application & Drug Control Authority (DCA) decision.
Step 3 : Regulatory OutcomeStep 4 : Post-registration Process
Maintenance of Registration, Withdrawal of Product Registration, Amendment to the particulars of the product, Post marketing activities
Maintenance of Registration, Withdrawal of Product Registration, Amendment to the particulars of the product, Post marketing activities
Step 4 : Post-registration Process







