Consumer Reporting of Side Effects
(Note: If you are a Healthcare Professional, please click here to report a medicinal problem or adverse drug reaction.)
If you, or someone you care for, experience any side effects to medicines or vaccines, please report to the NPRA by clicking the tab below. Your reports help improve the safe use of medicines in Malaysia.

| ConSERF Consumer Side Effect Reporting Form [Online Reporting] |
|
To report a suspected side effect or adverse drug reaction (ADR) to any medicine (including prescription, over-the-counter, traditional product, health supplement, etc.) or adverse event following immunisation (AEFI) to any vaccine.
For more information, please see the following: |
A side effect is defined as any unintended effect of a medicine which occurs at the normal dose used. Please report any side effect you find troubling, even if you are not certain it is due to the medicine or vaccine.
For further information on Vaccine Safety, please click on the images below.
How to Report?
You may report directly to the NPRA using the ConSERF form above. This form is used to report a suspected side effect to any medicine (prescription, over-the-counter, traditional products, health supplement, etc.) or to any vaccine.
(i) Submit your report directly online, OR
(ii) Download and print out the form, complete the details yourself, and submit it to us by email or post using the address stated below.
Please provide as many details as possible to ensure your report is useful. Your details will remain confidential and will not be passed on to any person outside NPRA without your permission. You will only be personally contacted if we need additional information related to your report.
You are encouraged to consult your healthcare provider (such as a doctor or pharmacist) before submitting your report. Your healthcare provider may be able to provide further clinical information based on your medical records that can help us evaluate your report.
For enquiries on reporting side effects or AEFI, please contact:
Centre for Compliance and Quality Control
National Pharmaceutical Regulatory Agency (NPRA)
Lot 36, Jalan Universiti,
46200 Petaling Jaya, Selangor.
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
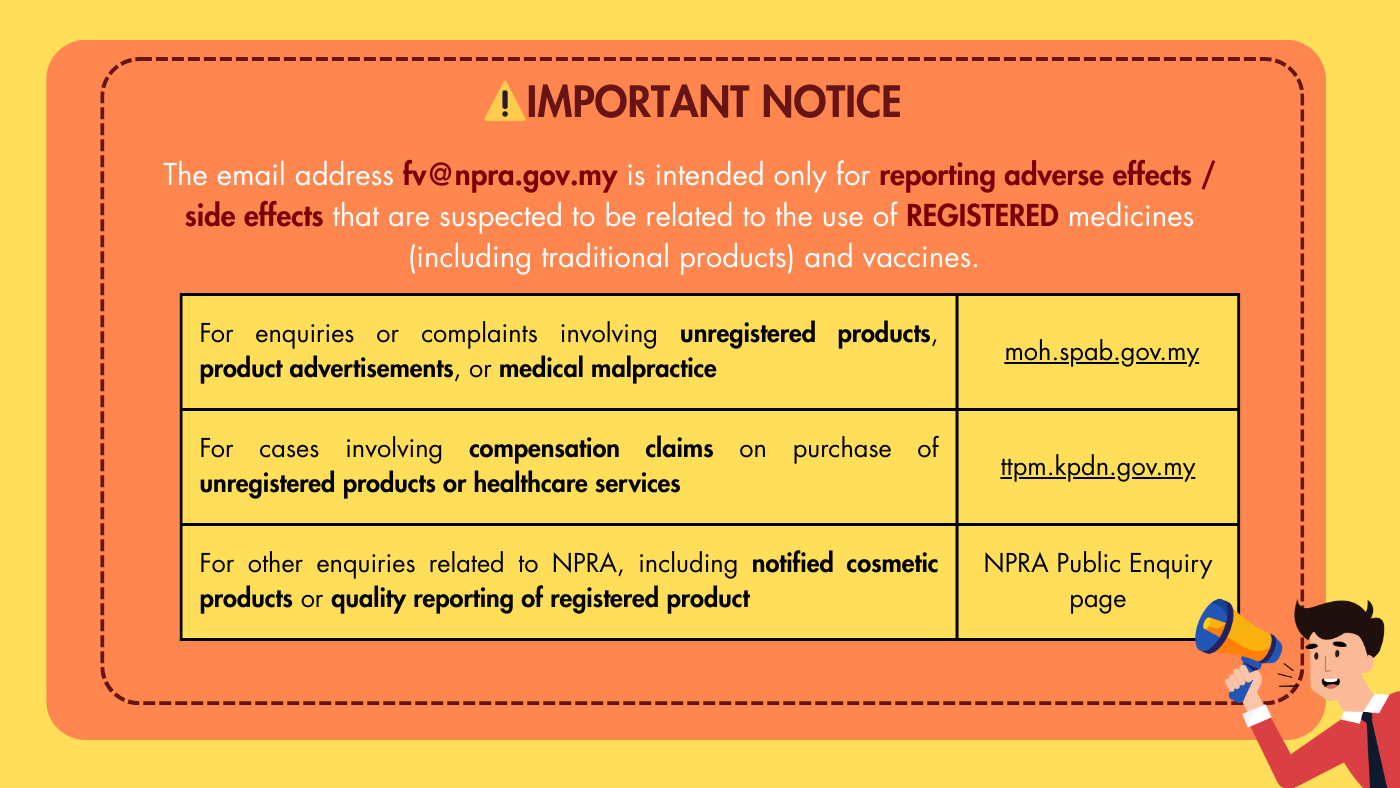
⚠️ Important Notice
The email address This email address is being protected from spambots. You need JavaScript enabled to view it. is intended only for reporting adverse / side effects that are suspected to be related to the use of REGISTERED medicines (including traditional products) and vaccines.
For enquiries or complaints involving unregistered products, product advertisements, or medical malpractice, please submit your report through the Ministry of Health’s Public Complaint Management System (SisPAA) at moh.spab.gov.my.
For cases involving compensation claims on purchase of unregistered products or healthcare services, claims may be submitted to the Tribunal For Consumer Claims Malaysia via https://ttpm.kpdn.gov.my/.
For other enquiries related to NPRA, including notified cosmetic products or quality reporting of registered products, please visit the NPRA Public Enquiry page.















