About the Malaysian Adverse Drug Reactions Advisory Committee
Introduction
- The Malaysian Adverse Drug Reactions Advisory Committee (MADRAC) was established under the Drug Control Authority (DCA) to perform pharmacovigilance activities for medicinal products registered in Malaysia.
- MADRAC provides DCA with important information pertaining to local and international drug safety issues, as well as advice on the management and communication of the risk following effective assessment of the benefit-risk profile of products.
- The National Centre for Adverse Drug Reaction Monitoring acts as the secretariat to MADRAC. It is a WHO-approved pharmacovigilance centre and was accepted as the 30th member of the World Health Organisation (WHO) Programme for International Drug Monitoring in 1990.
- Under this programme, all adverse drug reaction reports received and assessed by MADRAC are forwarded to the central WHO Global ICSR (Individual Case Safety Report) database, which is maintained by the Uppsala Monitoring Centre (UMC), the WHO Collaborating Centre in Sweden.
MADRAC Core Functions
- To promote ADR reporting in Malaysia.
- To provide reliable information and advice to DCA on drug safety.
- To disseminate drug safety information to doctors, pharmacists and other healthcare professionals.
- To participate in global pharmacovigilance activities via the WHO Programme for International Drug Monitoring.
Objectives of ADR Monitoring
- To detect adverse reactions to drugs as early as possible, especially for serious, rare or unknown reactions.
- To establish the frequency and incidence of adverse reactions for both well-recognised and newly discovered reactions.
- To identify risk factors that may predispose / induce / influence the development, severity and incidence of adverse reactions.
- To maintain a local database for sharing of information with regard to drug safety.
- To implement preventive measures to reduce the risks associated with drug use.
Reporting Mechanism
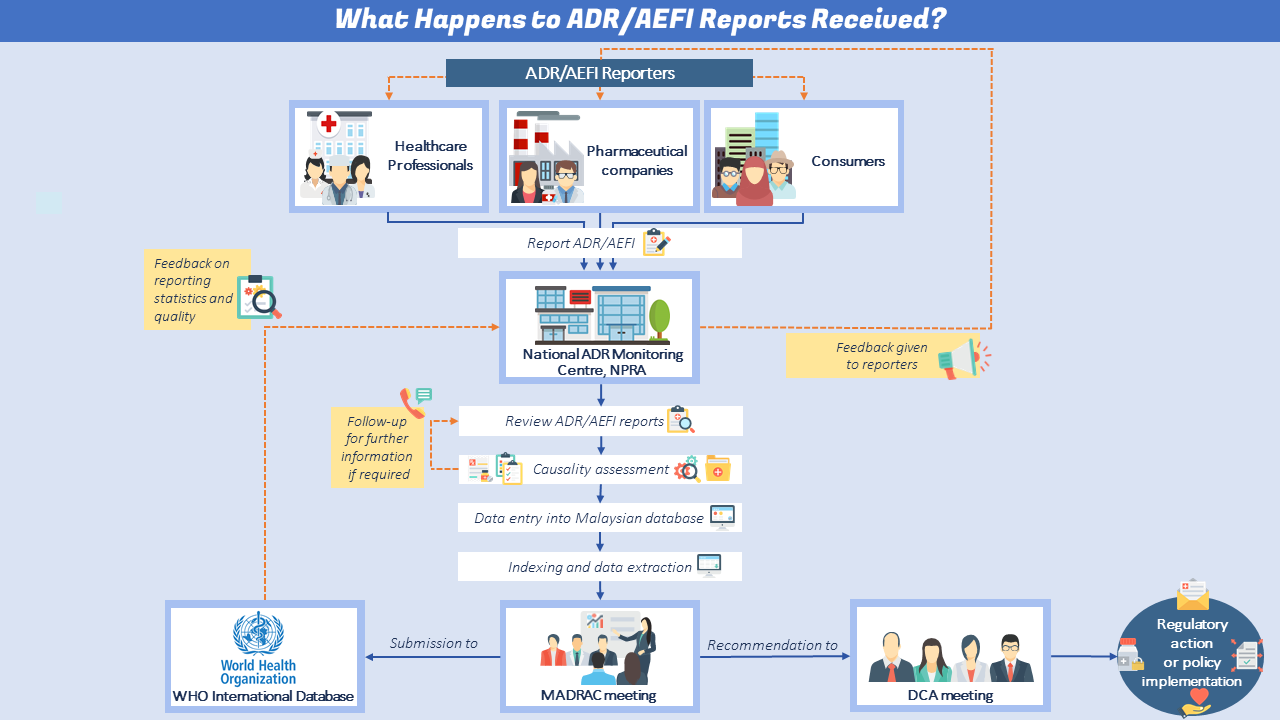
Impact of ADR Monitoring
Achievement of the primary objectives mentioned above will allow:
- Product registration holders to initiate steps to make changes to the product information.
- The regulatory authority to take appropriate action in the interest of public health to minimise risk as a result of medicine use.
- Healthcare professionals to have better awareness on the benefits and risks of medicines and thus practise rational prescribing.
- Consumers to be equipped with better knowledge of the consequences of drug use and thus use drugs more appropriately.










