Posted Date : 06 March 2013
Category : Other News and Important Announcements
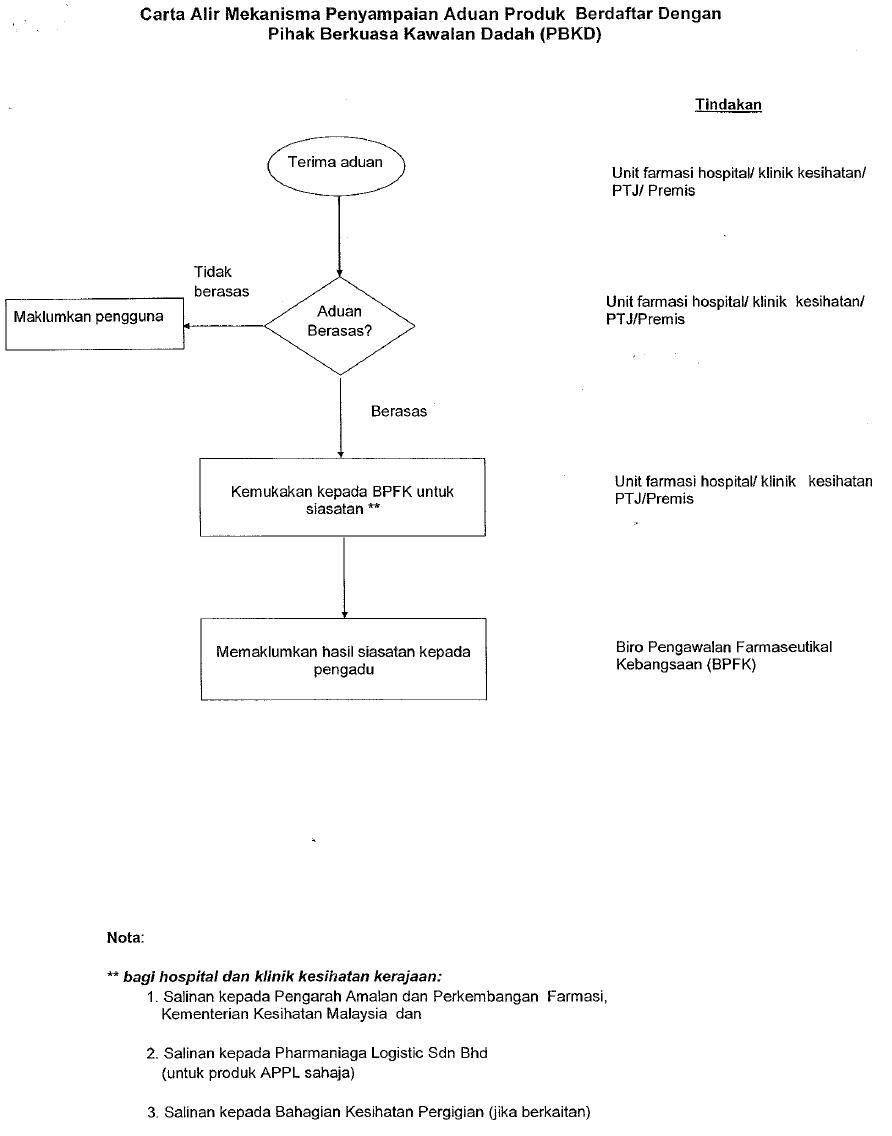
Click here to download the complete details.
Click here to download Borang Pelaporan Kesan Advers Ubat dan Vaksin
(![]() | 406KB) Updated on 5th August 2020
| 406KB) Updated on 5th August 2020
Click here to download Borang Pemantauan Kesan Sampingan Ringan Susulan Imunisasi
(![]() | 363KB) Updated on 5th August 2020
| 363KB) Updated on 5th August 2020