Natural Product
Answer: Yes. Natural products are subject to be tested prior to registration. The tests conducted are uniformity of weight and disintegration for tablets, capsules & pills and safety tests which include heavy metal and microbial contamination tests.
2. What are the requirements for natural product registration?
Kindly refer to the Drug Registration Guidance Document (Appendix 7 -Guideline on Registration of Natural Products) for the required registration documents.
3. What will happen if the dosing and indication claimed for products undergoing evaluation are not sufficiently supported by any reference?
Answer: The application will be rejected.
Frequently Asked Questions (FAQ) about the Guideline on Natural Products with Modern Claim
Introduction
This document is a compilation of Questions and Answers (Q&As) that provide clarifications and references to the industry so that readers can better understand the Guideline on Natural Products with Modern Claim.
This guidance applies to products intended for the Malaysian market. This document may be revised from time to time to include new Q & A’s or update information if changes are made to the ASEAN guidelines.
Questions & Answers
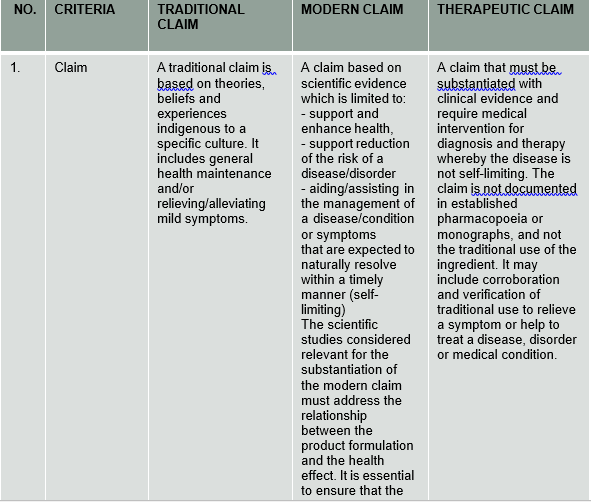
1. What are the differences between traditional claim, modern claim and therapeutic claim for natural products?
Traditional claim is a claim based on the sum total of knowledge, skills, and practices based on theories, beliefs, and experiences indigenous to a specific culture, used in the maintenance of health and prevention, diagnosis, improvement, or treatment of physical and mental illness.
Modern claim is a claim based on scientific evidence which is limited to supporting and enhancing health, risk reduction, or aiding/assisting in the management of a disease/condition or symptoms that are expected to naturally resolve within a timely manner (self-limiting) The scientific studies considered relevant for the substantiation of the modern claim must address the relationship between the product formulation and the health effect. It is essential to ensure that the level of claims permitted for modern claims does not surpass the allowable level of claims for therapeutic claims.
Therapeutic claims are claims that must be substantiated with clinical evidence and will require medical intervention for diagnosis and therapy whereby the disease is not self-limiting. The claim is not documented in established pharmacopoeia or monographs, and not the traditional use of the ingredient. It may include corroboration and verification of traditional use to relieve a symptom or help to treat a disease, disorder or medical condition.
Note: Kindly refer to table of comparisons at the end of this FAQ’s for further information.
2. Is prescription required for natural products with modern claim?
No prescription is required for natural products with modern claim.
3. Are there any differences in the formulation used in natural products with traditional claim, modern claim and therapeutic claim?
The allowable form of substances for active ingredient differ between the three types of claim. For traditional claim, the formulation can be in the form of crude (unextracted), or crude extract form.
For modern claim, the formulation must be in the form of quantified extracts. Formulation in the form of standardized extracts are accepted as well.
For therapeutic claim, the formulation must be in the form of standardized extracts.
4. Why active ingredients for natural products with modern claim must be in the form of quantified extracts?
For modern claim, references (scientific studies) must be submitted in order to address the relationship between the product formulation and the health effect. Herbal extracts used in studies will be in quantified or standardized extract to ensure reproducibility of results. Similarly, during production, quantified or standardized extract will minimise variations in the formulation and ensure batch to batch consistency in order to support the claim based on the references provided.
5. In the guideline, natural products with modern claim refers only to formulation containing herbal preparations in the form of quantified However, natural products are not limited to only herbal based products. Is it possible for formulation containing non-herbal preparations (eg animal, fungi, mineral) to be registered under this product category?
Currently, the scope of this guideline focuses on herbal products in the form of quantified extracts. This is because there are limited data on quantification of active markers for other natural sources (eg: mineral, fungi, animal) ingredients. However, if there are products that can conform to the requirements of the guideline, application may be submitted for evaluation.
6. Is it compulsory to submit toxicity data for all natural products with modern claim?
Toxicity data is required if there is no evidence of the documented history or traditional use of the ingredients. If toxic effects have been reported or there is insufficient documented safety evidence and there are doubts concerning the product/active ingredient, submission of toxicity data is also required.
7. Can toxicity data based on the ingredient(s) instead of the finished products be provided?
Toxicity data for each ingredient of the product shall be provided as supporting references if there is no toxicity data for the finished product. However, the ingredient(s) used in the cited literature must be the same with the intended product in terms of the species, part used, preparation method, extraction solvent, extraction parameters and concentration method.
8. Is there any specific duration of toxicity studies that is accepted under this natural products with modern claim category?
The quoted literature of toxicity studies should include duration of study that covers the duration of use of the claim. The durations of toxicity studies to support marketing for different treatment durations are outlined in the ICH M3 (R2) guideline.
9. Can scientific data on active ingredient(s) be used to substantiate natural products with modern claim?
For modern claim, only scientific studies (clinical study) conducted on the finished product or formulation
with a similar dosage regimen, dosage form, route of administration and target population should be submitted.
10. The dosage form used in the clinical study does not match the intended Is it acceptable? [Will clinical study findings with difference in formulation such as dosage form will be accepted?] For example, findings from a clinical study conducted on capsules containing 100mg of tongkat ali extract to be used to support a claim for finished product of 100mg of tongkat ali in tablet form.
Studies using single quantified extract with different dosage form may be considered if additional information such as pharmacokinetic data is provided to support the product used in the literature/trial has the same profile with the proposed product for registration.
11. Can the product claims be solely based on the benefit/efficacy of the active ingredient(s) in the product? Can a company make more than one claim for each active ingredient in the product, assuming every claim is adequately substantiated by evidence?
The intended use of the natural products with modern claim should be based on the finished product. The scientific studies considered relevant for the substantiation of the modern claim must address the relationship between the product formulation and the health effect. Claims solely based on the benefits or efficacy of the individual active ingredient(s) in the product is not acceptable.
12. What are some of the prohibited modern claims? For example, is the claim to treat diabetes allowable for natural products with modern claim?
All claims shall be consistent with the definition of natural products with modern claim. Proposed claims for this category shall only be limited for management, supporting and enhancing health, risk reduction or aiding/assisting in the management of a disease/condition or symptoms that are expected to naturally resolve within a timely manner (self-limiting).
Modern claims are not allowed to imply treatment, prevention or diagnosis of all diseases or medical conditions, including 20 diseases as stipulated in Section 3 in the Medicines (Advertisement and Sale) Act 1956 (Revised 1983) ;
- diseases or defects of the kidney,
- diseases or defects of the heart,
- diabetes,
- epilepsy or fits,
- paralysis,
- tuberculosis,
- asthma,
- leprosy,
- cancer,
- deafness,
- drug addiction,
- hernia or rupture,
- diseases of the eye,
- hypertension,
- mental disorder,
- infertility,
- frigidity,
- impairment of sexual function or impotency,
- venereal disease,
- nervous debility or other complaint or infirmity arising from or relating to sexual intercourse.
13. Specification limit is not stated for certain test of active ingredient and/ or finished What are the limits accepted by NPRA?
The specifications are based on the standard testing method used. However, there are also examples provided in Appendix 7: Guideline on Registration of Natural Products, DRGD. For heavy metal limit and microbial contamination test, specific limit for finished product as in Appendix 7 Guidelines on Registration of Natural Products can be adopted. For other acceptance limit, it must be determined by the manufacturer. The reference used for the specifications must be stated in the COA or related documents. If there is specific monograph in pharmacopoeia for the herbal substances / preparation / quantified extracts/products, the acceptance limit in the monograph should be followed. Justification must be provided if the specification is different from the monograph.
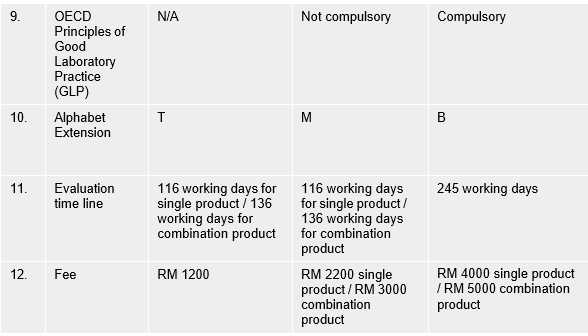
14. Will the same product registration number be used for natural products with modern claims?
The product registration number for natural products is differentiated by the type of claim with the use of different suffixes as shown below:
Traditional Claim: MALxxxxxxxxT Modern Claim: MALxxxxxxxxM Therapeutic Claim: MALxxxxxxxxB
15. Can a product with the same formulation be registered under two different categories i.e natural product with traditional claim and natural products with modern claim?
A product is allowed to register under one category only. If there is a need to change category for current registered product, applicant may proceed for registration as replacement product.
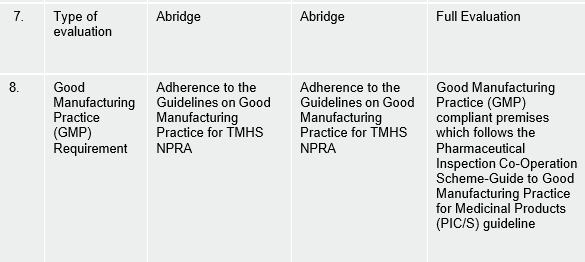
Table of comparison between 3 categories of Natural Products