Frequently Asked Questions : Compliance & Licensing
The measures to be taken during storage, transportation, and distribution of registered products / notified cosmetics and their related materials to ensure the quality and integrity of the intended use are preserved when they reach consumers.
GDP can serve as a guide for parties involved in the supply chain of registered products / notified cosmetics to maintain the quality and integrity of registered products/notified cosmetics at all levels of the supply chain.
All holders of Manufacturer’s License / Import License / Wholesaler’s License are required to fulfil:
1. The license condition is to comply with GDP requirements.
2. The directive from the Senior Director of Pharmaceutical Services to manufacturers / importers / wholesalers of registered products / notified cosmetics has been in effect since 1 January 2012 to comply with GDP requirements as stipulated in the Guidelines on Good Distribution Practice, Malaysia.
1. Guideline on Good Distribution Practice
2. Supplementary Notes on Annex 1: Management of Time and Temperature Sensitive Products (TTSP) of Guideline on Good Distribution Practice
Inspection of the Import License and the Wholesaler’s License holders to verify compliance with the Guideline on Good Distribution Practice.
Import License and Wholesaler’s License holders.
The officers from NPRA and/or the Pharmacy Enforcement Division.

The NPRA will determine the mode of inspection based on a risk assessment that has been conducted.
GDP inspection will be conducted either (a) on-site or (b) remotely:
a) On-Site Inspection
A physical inspection conducted at the business premises and/or store address(es) of registered products / notified cosmetics.
b) Remote Inspection
A virtual inspection involving the evaluation of documentation and a live virtual tour of the premise(s) and/or store(s) via an agreed-upon online communication platform.
The inspection scope will encompass compliance aspects based on the Guideline on Good Distribution Practice. A general review of the inspection scope can be found in Annex 2: General Points to Consider for Auditee.
Based on the findings of the inspection, the following levels of GDP compliance will be determined:
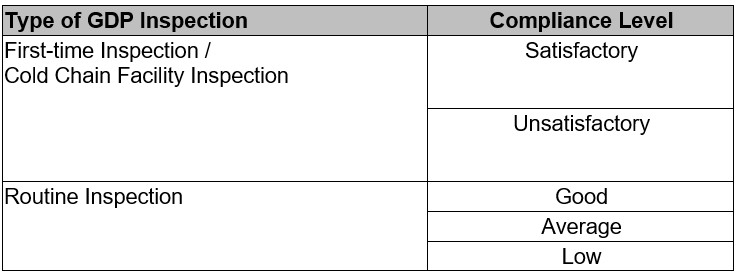
The duration of time taken for each GDP inspection depends on the scope and activities related to GDP under the responsibility of the holder of the Import License and the Wholesaler’s License.
The GDP inspection will be conducted periodically, and the determination of the inspection frequency is based on a risk matrix established by NPRA covering the current level of GDP compliance, the scope of GDP activities, and the types of products handled.
Regulatory punitive actions can be imposed on companies that fail to comply with GDP requirements.
License application for year 2024 and earlier
Not necessary. The company can proceed to apply for an Import License / Wholesaler's License, and NPRA will schedule the GDP inspection at the company's premises later.
License application for year 2025 and later
Under the enhanced licensing process, a GDP inspection may be required following submission of a new Import License / Wholesaler’s License application, subject to predefined criteria. Applicants must be fully prepared for documentation review and an inspection as part of the evaluation process.
The following steps depend on the outcome of the inspection:
If the outcome is Pending CAPA:
The company must submit a satisfactory Corrective and Preventive Action (CAPA) report to NPRA within six (6) months from the inspection date. Failure to submit satisfactory CAPA report within this period will result in the inspection being deemed Unsatisfactory.
If the outcome is Satisfactory (or Satisfactory after CAPA):
The company may apply for updating the License Conditions for handling cold chain products to the Licensing and Certification Section, Centre of Regulatory Coordination and Strategic Planning, NPRA. Please refer to the Surat Makluman Penambahbaikan Proses Pengeluaran Lesen Mengimport / Lesen Pemborong Melalui Penilaian dan Pemeriksaaan Pra-Pelesenan, effective from 27 March 2024.
Cold Chain Facility Inspection details for the company can also be found on the Cold Chain Facilities List.
If the outcome is Unsatisfactory (or Unsatisfactory after CAPA):
The company may reapply for the Cold Chain Facility Inspection six (6) months after the previous inspection date, subject to consideration of the CAPA implemented.
This period allows the company to have sufficient time to implement comprehensive CAPA to address all identified deficiencies. Effective CAPA resolution is crucial to ensure the company is fully prepared to handle cold chain products, thereby safeguarding the product’s quality, safety and efficacy.
Yes.
The GDP inspection also applies to third parties appointed by the company to handle the storage, transportation and/or distribution of its registered products / notified cosmetics.
No.
The company may refer to Appendix 32: Explanatory Notes for Repackers of the Drug Registration Guidance Document (DRGD) and/or contact the Good Distribution Practice Section, Centre of Compliance and Quality Control, NPRA.
The NPRA generates reports highlighting common deficiencies found during GDP inspections. These reports assist stakeholders in identifying and addressing compliance issues to improve their practices.
Remote Inspection offers flexibility while maintaining the effectiveness of the inspection process, even in challenging circumstances. This method has been successfully used by NPRA during the COVID-19 pandemic, and will now be implemented as an alternative to on-site inspections.
Its implementation also aligns with the enhanced licensing process, which introduced pre-licensing evaluation and inspection.
Companies / license holders must:
• Provide consent to participate in the Remote Inspection.
• Submit required documents similar to those reviewed during On-Site Inspections.
• Ensure access to stable internet and virtual communication tools.
• Prepare their premises for a virtual tour.
Phone: +603-78835400 or Staff Directory
Official Email: saeb@npra.gov.my
Official Portal: https://npra.gov.my
Updated 19 December 2024








