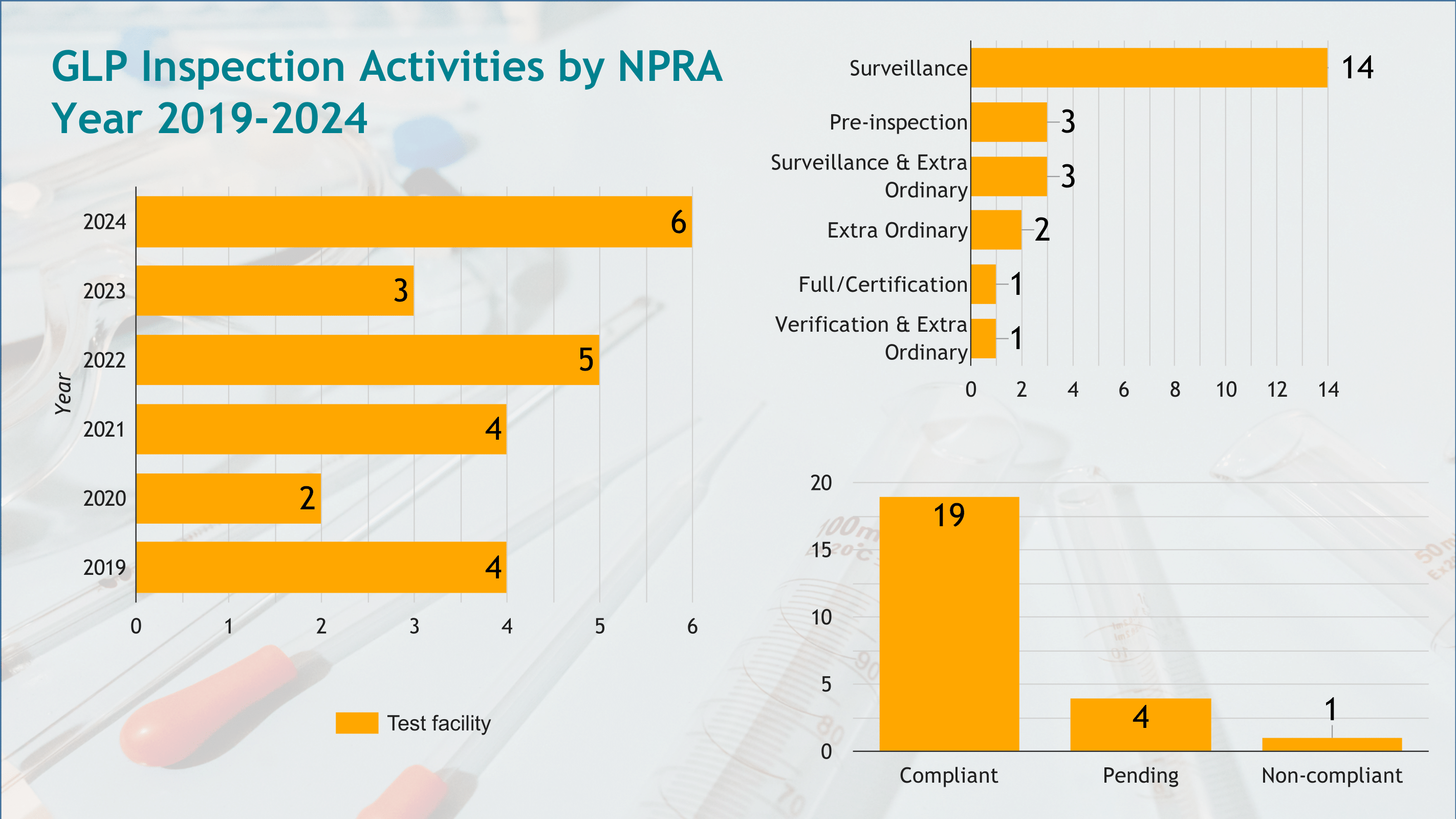
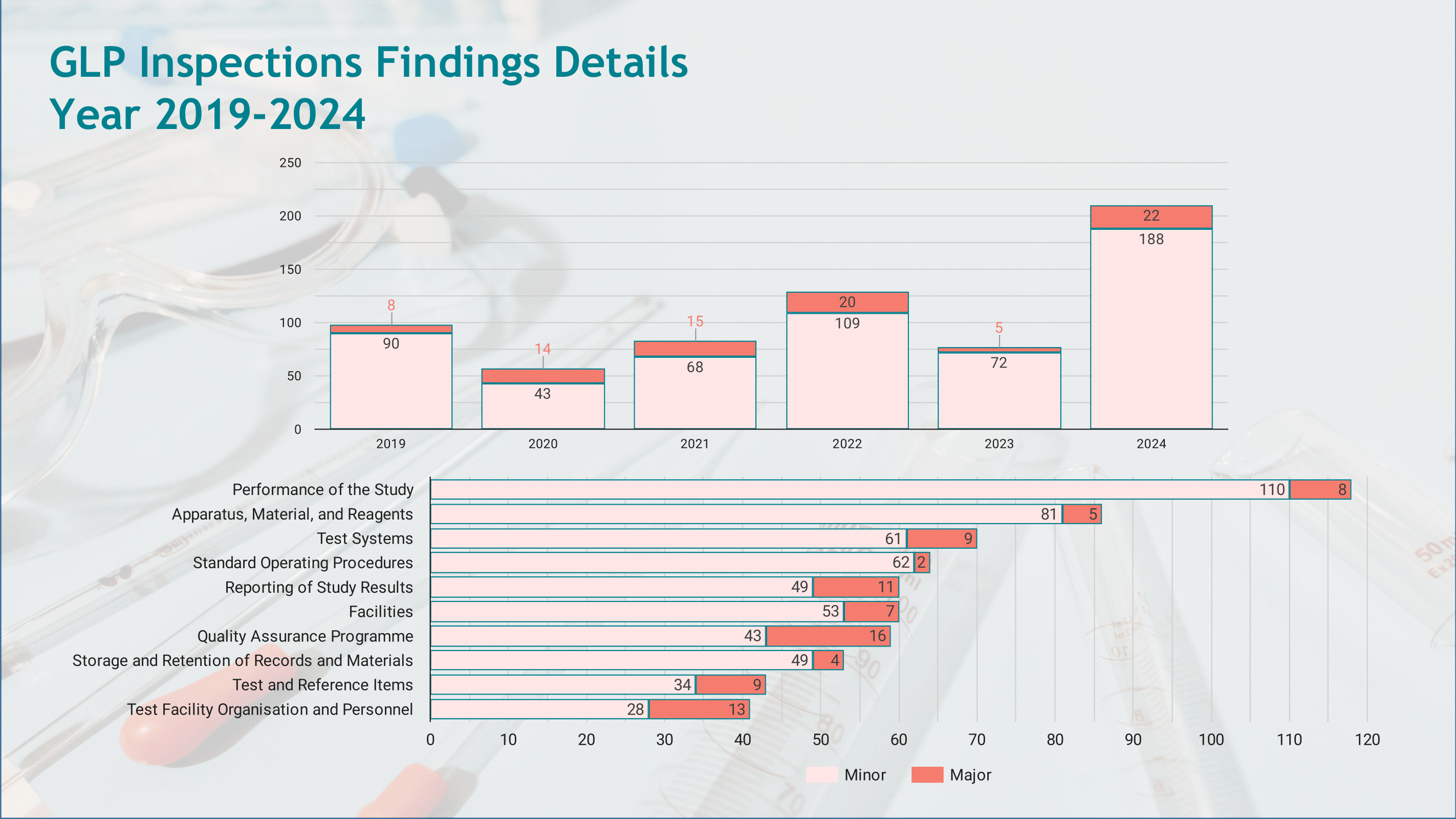
GLP Inspection Activity
As one of Malaysia's appointed Compliance Monitoring Authorities (CMAs), NPRA oversees adherence to the Organisation for Economic Co-operation and Development (OECD) Principles of Good Laboratory Practice (GLP) through its GLP Compliance Monitoring Programme (CMP).
The daily management of this programme is conducted by the Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) Section, part of the Centre for Compliance and Quality Control. The Key GLP Activities include:
- Conducting inspections of test facilities to assess compliance with OECD GLP Principles.
- Issuing GLP compliance certificates for facilities meeting the required standards.
- Reporting inspection outcomes, including compliance status and key findings, to the OECD GLP Secretariat.
The infographic provides an overview of the inspections performed, the compliance statuses of inspected facilities, and a summary of findings, ensuring transparency and alignment with international GLP standards.