DISCLAIMER: This publication is aimed at health professionals. The information is meant to provide updates on medication safety issues, and not as a substitute for clinical judgement. While reasonable care has been taken to verify the accuracy of the information at the time of publication, the NPRA shall not be held liable for any loss whatsoever arising from the use of or reliance on this publication.
Trigger of Signal
This signal review was triggered by the notable disproportion in the reporting of fixed drug eruption (FDE) associated with paracetamol (n=99; IC025=1.5).1,2 Although this association is known and warnings on severe skin reactions have been included in the product information, the upward trend of reports in recent years, particularly involving paediatric patients, has raised concern.
Thus, this review was initiated to assess the characteristics of FDE reported in patients aged below 12 years and to determine whether further risk minimisation measures are necessary.
Background
Paracetamol, also known as acetaminophen, is a commonly used over-the-counter (OTC) drug for mild to moderate pain and pyrexia.3 It has been on the market for nearly 70 years and is generally considered well-tolerated and safe.4 However, like any other drug, adverse drug reactions (ADRs) have been reported with paracetamol. Cutaneous reactions, including FDE, are among the most frequently reported ADRs following the use of paracetamol.5
FDE, a delayed type 4 hypersensitivity reaction, is a distinctive skin reaction characterised by the development of one or more annular or oval erythematous patches that recur at the same site upon systemic re-exposure to the causative drug.6-10 FDE was reported to preferentially involve the limbs, face, trunk, genitalia, or lips. Paracetamol is one of the most common causes of FDE.6,8,11 Other common causative drugs include nonsteroidal anti-inflammatory agents (NSAIDs) and antibiotics.
NPRA previously reviewed the safety of paracetamol in 2015, resulting in the issuance of a Drug Control Authority (DCA) directive and communication of this risk in the MADRAC Bulletin (August 2015 edition).12-13 The DCA Directive mandates that all products containing paracetamol include an allergy alert in their outer label, package insert (PI), and consumer medication information leaflet (RiMUP), as follows:
Local Reports2
As of March 2024, NPRA has received a total of 99 reports of FDE associated with paracetamol, of which 41 reports were in patients aged below 12 years. Figure 1 illustrates the reporting trend.
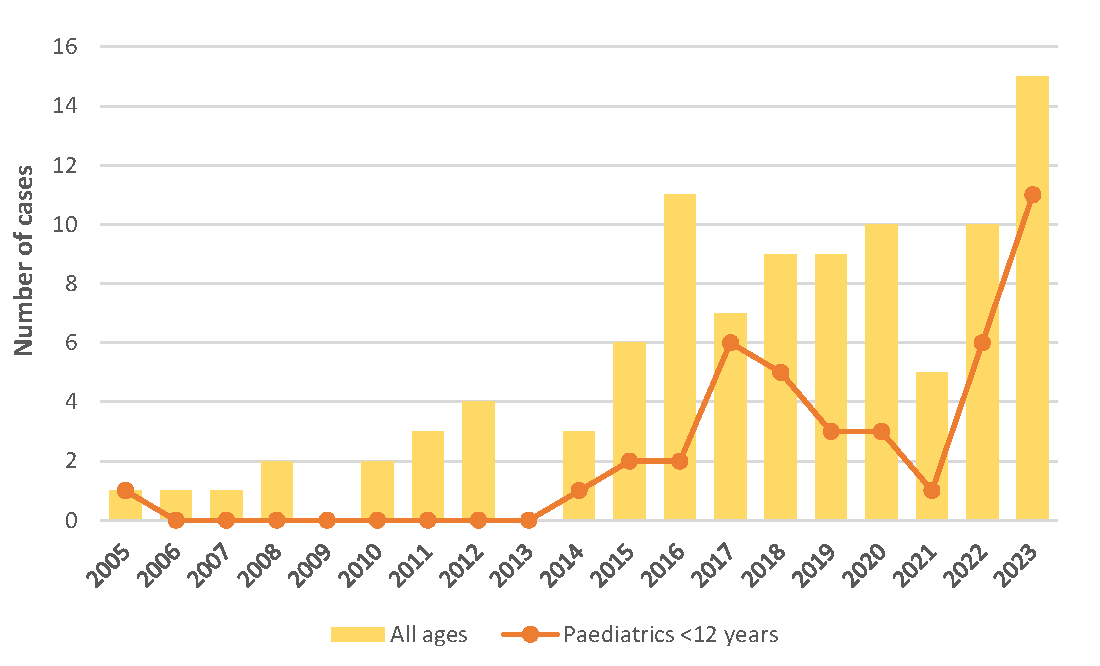
Figure 1. Fixed drug eruption (FDE) cases associated with paracetamol in the Malaysian National ADR Database, by year (n=99)
Among these 41 cases, majority were males (68%) and 8 cases were reported as serious, leading to hospitalisation or prolonged hospitalisation. A close temporal relationship was evident in several cases. Among cases reporting time-to-onset, the majority (19/20; 95%) indicated that the first symptoms appeared within 24 hours after taking paracetamol, with 13 cases experiencing symptoms within 10 hours. Recovery was reported in majority of cases (78%), while one case reported non-recovery at the time of reporting, and the remaining cases had an unknown outcome. The reports were assigned causality C1 certain (5%), C2 probable (5%), or C3 possible (90%).
Although the number of FDE cases reported with paracetamol is relatively small compared to its widespread usage, the significance arises from the fact that 41% of cases involved paediatric patients below 12 years old, and 20% of these were categorised as serious reactions. This underscores the importance of taking these reports seriously and highlights the need for risk minimisation measures.
Conclusion & Advice
Following this review, the Malaysian Adverse Drug Reactions Advisory Committee (MADRAC) concluded that risk communication is necessary. NPRA has disseminated two infographics, as follows:
(i) For the general public, alerting them to stop taking paracetamol and seek medical advice at the early signs and symptoms of skin reactions (available in both in English at e-Brochures and Malay at e-Risalah);
(ii) For healthcare professionals, highlighting the increasing trend of FDE reports associated with paracetamol (available in English at e-Brochures).
FDE can occur in all age groups, including paediatric patients. Avoidance of the causative drug is important to prevent FDE recurrence.6,14
As an OTC drug, paracetamol is easily accessible to consumers. The general public may also face difficulties in identifying products which contain paracetamol due to the various combinations and formulations available.15-16 Considering these issues, healthcare professionals are encouraged to:
- Educate patients on how to identify paracetamol as the active ingredient in single and combination products in various formulations, as well as on the proper use of this medicine.
- Remind patients to seek medical assistance immediately if they or their children experience any of the following signs and symptoms: skin reddening, blisters, or rash after taking paracetamol.
- Report any cases of skin reactions, including FDE, suspected to be associated with the use of paracetamol to the NPRA.
References:
- Uppsala Monitoring Centre (UMC). The WHO Global ICSR Database (VigiLyze) [Internet]. 2024 [cited 2024 Apr 1]. Available from: https://www.vigilyze.who-umc.org (access restricted).
- National Pharmaceutical Regulatory Agency (NPRA). The Malaysian National ADR Database (QUEST3+) [Internet]. 2024 [cited 2024 Apr 1]. Available from: https://www.npra.gov.my (access restricted).
- Pharmaceutical Services Programme. Paracetamol [Generic Name]. Formulari Ubat KKM (FUKKM). 2024 [cited 2024 Apr 1]. Available from: https://pharmacy.moh.gov.my/
- Freo U, Ruocco C, Valerio A, Scagnol I, Nisoli E. Paracetamol: a review of guideline recommendations. Journal of clinical medicine. 2021 Jul 31;10(15):3420. Available from: https://doi.org/10.3390/jcm10153420
- Nagai J, Uesawa Y, Shimamura R, Kagaya H. Characterization of the adverse effects induced by acetaminophen and nonsteroidal anti-inflammatory drugs based on the analysis of the Japanese adverse drug event report database. The Clinical Journal of Pain. 2017 Aug 1;33(8):667-75. Available from: https://doi.org/10.1097/AJP.0000000000000457
- McClatchy J, Yap T, Nirenberg A, Scardamaglia L. Fixed drug eruptions–the common and novel culprits since 2000. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2022 Oct;20(10):1289-302. Available from: https://doi.org/10.1111/ddg.14870
- Ben Fadhel N, Chaabane A, Ammar H, Ben Romdhane H, Soua Y, Chadli Z, Zili J, Boughattas NA, Ben Fredj N, Aouam K. Clinical features, culprit drugs, and allergology workup in 41 cases of fixed drug eruption. Contact Dermatitis. 2019 Nov;81(5):336-40. Available from: https://doi.org/10.1111/cod.13351
- Heng YK, Yew YW, Lim DS, Lim YL. An update of fixed drug eruptions in Singapore. Journal of the European Academy of Dermatology and Venereology. 2015 Aug;29(8):1539-44. Available from: https://doi.org/10.1111/jdv.12919
- Jung JW, Cho SH, Kim KH, Min KU, Kang HR. Clinical features of fixed drug eruption at a tertiary hospital in Korea. Allergy, asthma & immunology research. 2014 Sep 1;6(5):415-20. Available from: https://doi.org/10.4168/aair.2014.6.5.415
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. International journal of dermatology. 1998 Nov;37(11):833-8. Available from: https://doi.org/10.1046/j.1365-4362.1998.00451.x
- Savin JA. Current causes of fixed drug eruption in the UK. British Journal of Dermatology. 2001 Oct 1;145(4):667-8. Available from: https://doi.org/10.1046/j.1365-2133.2001.04422.x
- National Pharmaceutical Regulatory Agency (NPRA). Direktif untuk produk yang mengandungi paracetamol, termasuk produk kombinasi : Pengemaskinian label, sisip bungkusan, dan risalah maklumat ubat untuk pengguna (RiMUP) dengan amaran berkaitan kesan advers serius pada kulit. [Internet]. 2015 June 3 [cited 2024 Apr 4]. Available from: https://npra.gov.my/images/Circulars_Directive/Regulatory_Information/Direktif%20Bil%205_2015_paracetamol.pdf
- MADRAC Newsletter. National Pharmaceutical Regulatory Agency (NPRA). Paracetamol: Risk of Serious Skin Reactions [Internet]. 2015 Aug [cited 2024 Apr 4]. Available from: https://www.npra.gov.my/images/Publications/Newsletter_MADRAC_Bulletin/Bulletin_MADRAC_Aug2015.pdf
- MyHealth Portal Ministry of Health Malaysia. Fixed Drug Eruption [Internet]. 2019 Aug 23 [cited 2024 Apr 4]. Available from: http://myhealth.moh.gov.my/en/fixed-drug-eruption-2/
- Chong CP, Tan SF, Chooi WT. Exploring consumers’ perceptions and knowledge of acetaminophen (paracetamol): a cross-sectional study from Penang, Malaysia. Int. J. Pharma Sci. Res. IJPSR. 2020;11:52-7.Available from: http://www.ijpsr.info/docs/IJPSR20-11-03-010.pdf
- Tan SF, Chong CP, Chooi WT. An evaluation of practices, perceptions and understanding about use of acetaminophen (paracetamol) among Malaysian consumers: A qualitative study. Malaysian Journal of Pharmaceutical Sciences. 2015;13(1):25-41. Available from: http://web.usm.my/mjps/mjps13012015/mjps13012015_3.pdf
Written by: Lee Sing Chet
Reviewed/Edited by: Nora Ashikin Mohd Ali, Dr Rema Panickar, Norleen Mohamed Ali










