Medicine Shortage & Discontinuation Reporting
Ensuring a consistent supply of essential medicines is a critical component of any healthcare system. Medicine shortages disrupt patient care, delay treatments, and pose increased health risks, particularly for vulnerable populations.
In May 2022, Malaysia experienced a significant medicine shortage crisis involving 54 active ingredients and affecting 1,384 pharmaceutical products, including six (6) antibiotics. This crisis, resolved by 1st July 2023, underscored the growing need for an effective and coordinated approach to monitoring, reporting, and managing medicine shortages to safeguard public health.
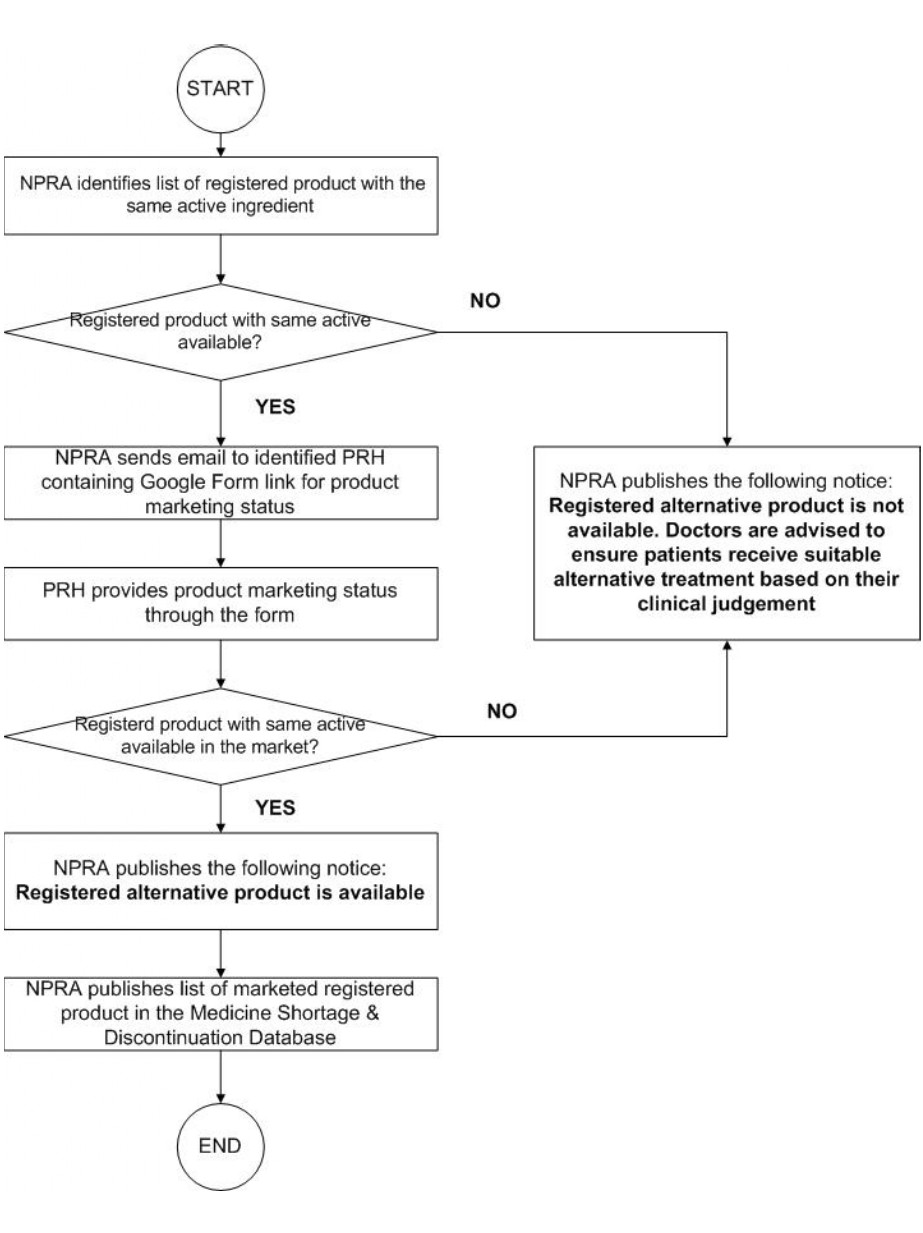
The purpose of this guideline is to provide clear and actionable instructions for Product Registration Holders (PRH) to detect, report, and manage current and anticipated medicine shortages efficiently as well as to report discontinuation of a product. It aims to strengthen Malaysia’s capacity to mitigate supply disruptions and reduce their impact on patients.
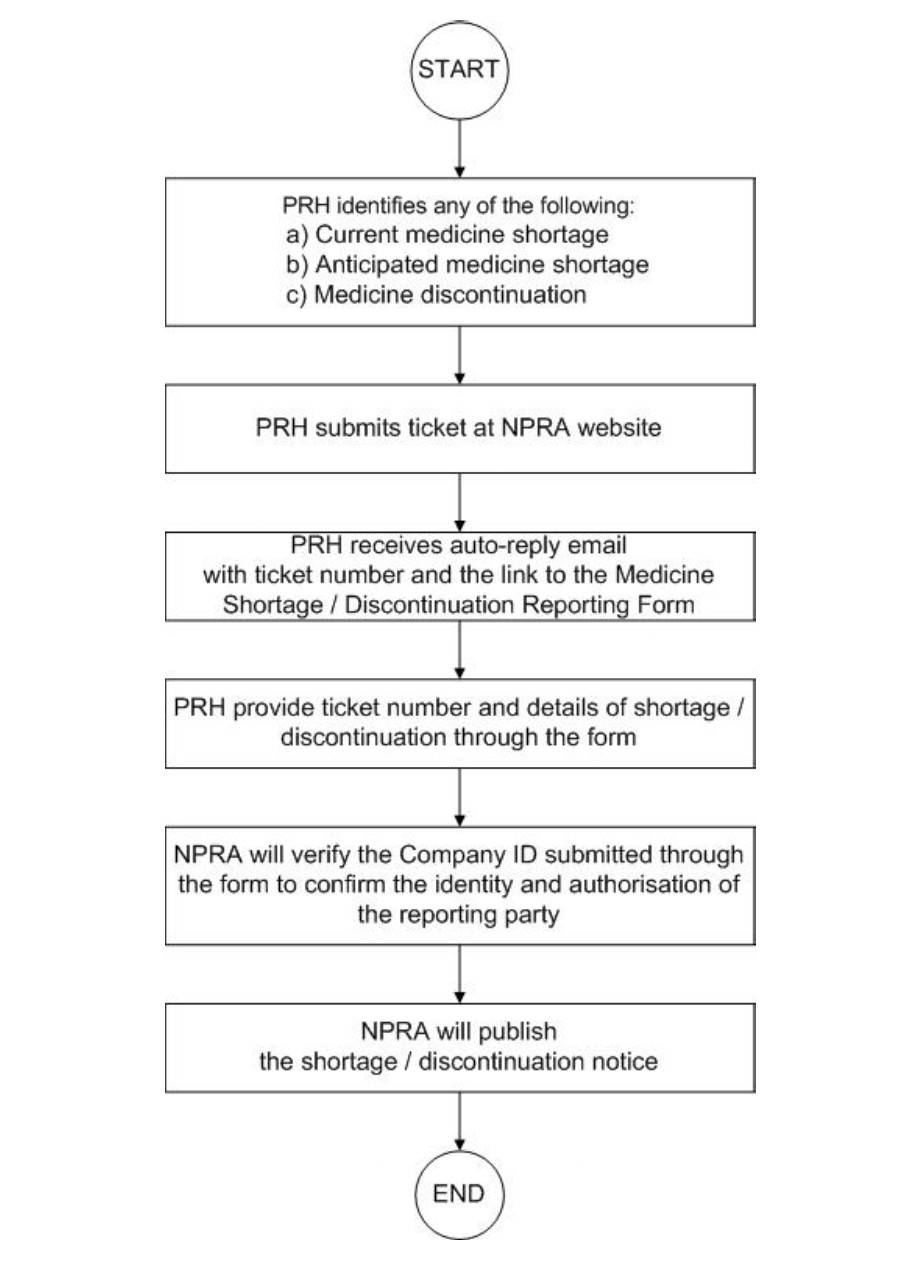
Reporting by Product Registration Holder (PRH) only:
1. First PRH need to submit ticket through this link2. Then fill in the google form (Medicine Shortage/Discontinuation Reporting Form) with reference number that you get from the ticket.