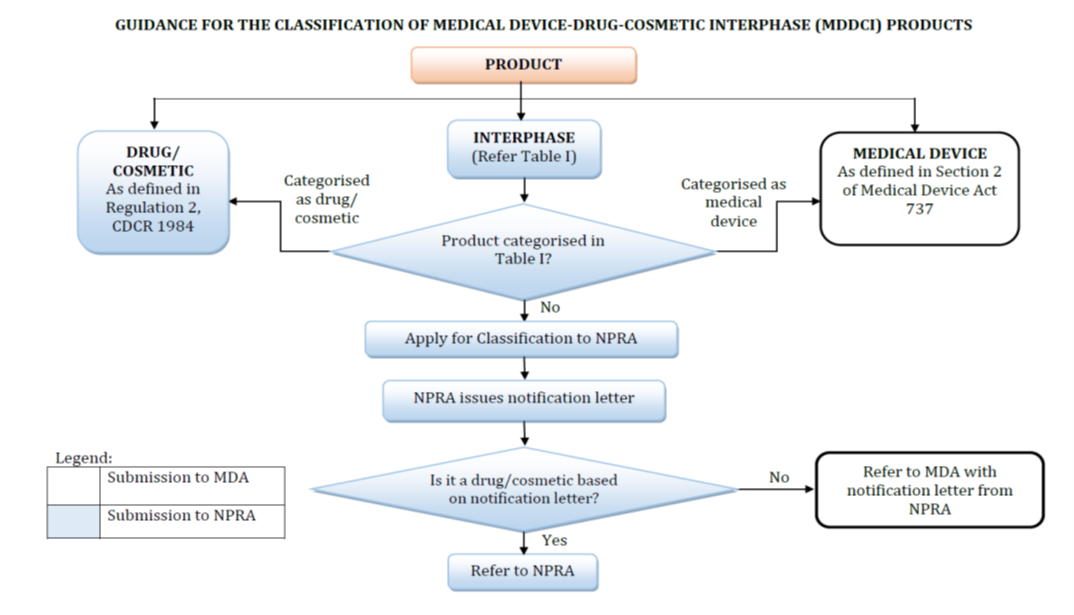
MEDICAL DEVICE - DRUG - COSMETIC INTERPHASE PRODUCTS
Medical Device-Drug-Cosmetic Interphase (MDDCI) Products are those products that are not clearly defined as a medical device or drug/cosmetic in accordance to the Medical Device Act 737, Control of Drugs and Cosmetics Regulations 1984 and Sale of Drugs Act 1952.
Registration of drug products/ notification of cosmetics that has been classified must follow the requirements that have been set forth as follows:
- Drugs & Cosmetics – The registration/ notification regulated by the NPRA is in accordance with the requirements set forth in the Poisons Act 1952 and its Regulations, Sales of Drugs Act 1952 and the Control of Drugs and Cosmetics Regulations 1984;
- Medical Device – The registration regulated by Medical Device Authority is in accordance with the requirements set forth in the Medical Devices Act 2012 (Act 737).
Combination products includes:
CLASSIFICATION CRITERIA
Medical device does not achieve its primary mode of action in or on the human body by pharmacological, immunological or metabolic means, but may be assisted in its intended function by such means.;
For classification of MDDCI products and combination products as decided by the committee, please refer to APPENDIX 2, Table I : SUMMARY OF MEDICAL DEVICE-DRUG-COSMETIC INTERPHASE (MDDCI) PRODUCT CLASSIFICATION DECISION (click here)