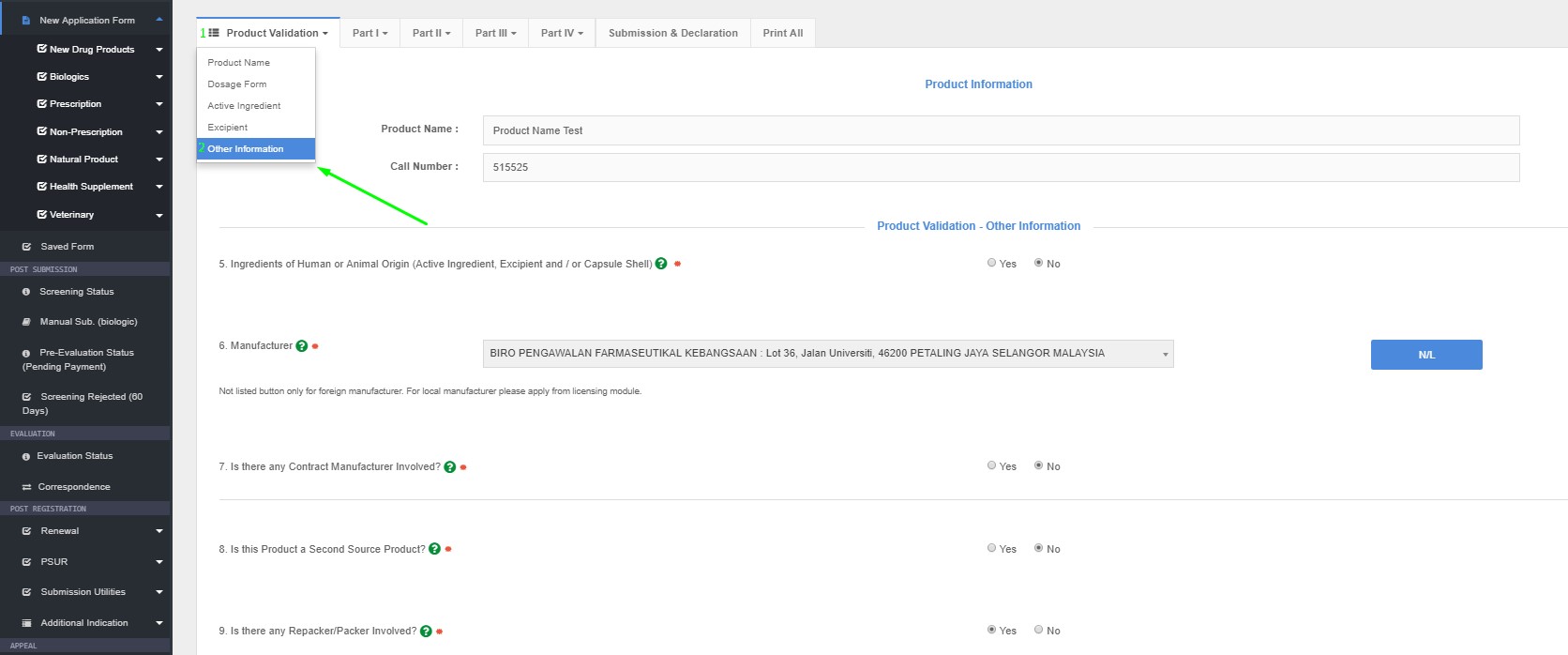
5. Ingredients of Human or Animal Origin (Active Ingredient, Excipient and / or Capsule Shell)
- 5.1 Origin
- 5.2 Function
- 5.3 In the Manufacturing Process
- 5.4 In the Final Product
- Please select YES if there is any active ingredient, excipient and/or capsule shell derived from human or animal.
- Please specify whether the origin is porcine/ bovine/ ovine or others.
- If OTHERS is selected, please specify the source.
6 Manufacturer
- Please select the manufacturer of the finished product from the database list. If the manufacturer is not listed, please click the ‘Not Listed’ button; upon which, the particulars of the manufacturer should be provided by applicant, along with the manufacturer’s GMP Certificate/ Status
- Please take note that the manufacturer must be listed in the database before applicant may proceed with product registration application.
- Please ensure the name and address of manufacturer is the same with the GMP certificate attached.
7. Is there any Contract Manufacturer Involved?
- Status as to whether the declared manufacturer is a contract manufacturer or otherwise, has to be entered. Click the appropriate button ‘Yes’ or ‘No’.
8. Is this Product a Second Source Product?
- An application for a second source may be considered where deemed necessary.
- Please select YES or NO if the product application is second source or otherwise.
- If the product is second source, a declaration letter stating that the product is second source, along with justification for second source must be provided. Applicant must also state the first source product by selecting from the database.
- Please refer: Appendix 8: 8.1.6 SECOND OR THIRD SOURCE
9. Is there any Repacker/Packer Involved?
If Yes, please state
- Manufacturer (repacker/packer) name
- Manufacturer (repacker/packer) address
- Certificate of Good Manufacturing Practice (GMP)
- Packaging Process
Please select YES or NO if the manufacturing of the product involves any repacker/packer. If a repacker is involved, please be advised that the repacker/packer must have a valid GMP Certificate to perform such activity.
10. Is the Product Manufactured for Export Only?
- Registration of product for export only (FEO) refers to locally manufactured products for exporting purpose only and not marketed locally. This does not apply to imported products meant to be packed/repacked locally and to be re-export.
- Please Refer :
11. Is the Product Under Patent Protection?
- Click the appropriate button ‘Yes’ or ‘No’.
- Please enter name of company and click button ‘search’ to select other manufacturer (repacker) listed in the database. For a new other manufacturer (repacker) which is not listed in the database search, please click ‘Not Listed Manufacturer’ button. Automatic e-mail will be send to NPRA for verification.
- Select from processing type drop-down list, e.g. assembly, packing, production, labelling, fill and finish, others.
12. Is this an Imported Product?
- *If Yes,
- 12.1 Section E Type
- Please select YES or NO, depending whether this is an imported product or otherwise. If imported product, please select Section E Type either Type A (CPP) or Type B (CFS & GMP).
13. Does this product contain any premix?
*If Yes,
- 13.1 State your Premix Form
- 13.2 Manufacturer Name
- 13.3 Manufacturer Address
- 13.4 GMP Certificate
- 13.5 Formulation Process
- 13.6 Manufacturing Process
- 13.7 Specification of Analysis
- 13.8 Certification of Analysis
Please select YES or NO, depending whether this product contains any premix or not. If it does, applicant should state the premix form, manufacturer name and address, and provide its GMP Certificate, Formulation Process, Specification of Analysis and Certificate of Analysis.
Note: Process Validation (Protocol & Report) for API Premix (in Section P3.4)
14. Is this A Replacement Product?
*If Yes,
- 14.1 Declaration Letter
- 14.2 Product(s) to be replaced
Please select YES or NO, depending whether this is a replacement product or not. If it is, applicant must attach a declaration letter and state the product(s) to be replaced with justification.
15. Does this product need Priority Review?
*If Yes,
- 15.1 Application Letter
- 15.2 Priority Review Status
- 15.3 Date of Grant
Please select YES or NO, depending if the product has priority review or otherwise. If there is priority review granted, applicant should click YES, and provide the Application Letter to NPRA, Approval Letter for Priority Review from NPRA, Priority Review Status and Grant Date.
16. Request for Data Exclusivity (DE)?
Not applicable to generic product.
*If Yes,
- 16.1 DCA Reference Country (for DE)
- 16.2 Date of Approval in Reference Country
- 16.3 Duration of DE Granted in Reference Country
- 16.4 Letter of Intent
17. Is this product Certified Halal? (Yes/No)
*If Yes,
- 17.1 Halal Certificate
- 17.2 Certificate No.
Not applicable to generic product.
18. Does this product contain a medical device component?
*If Yes,
- 18.1 Medical Device
- Please select YES or NO, depending whether this product contain medical device component or not. If YES, applicant should attach the medical device component document.
Please refer :
- Directive No. 4 Year 2017, Ref. (9) dlm.BPFK/PPP/07/25 Jld. 1 : DIREKTIF KUATKUASA PEMAKAIAN GUIDELINE FOR REGISTRATION OF DRUG-MEDICAL DEVICE AND MEDICAL DEVICE-DRUG COMBINATION PRODUCTS
- Guideline For Registration Of Drug-Medical Device and Medical Device-Drug Combination Products
How to Access Other in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Other Information