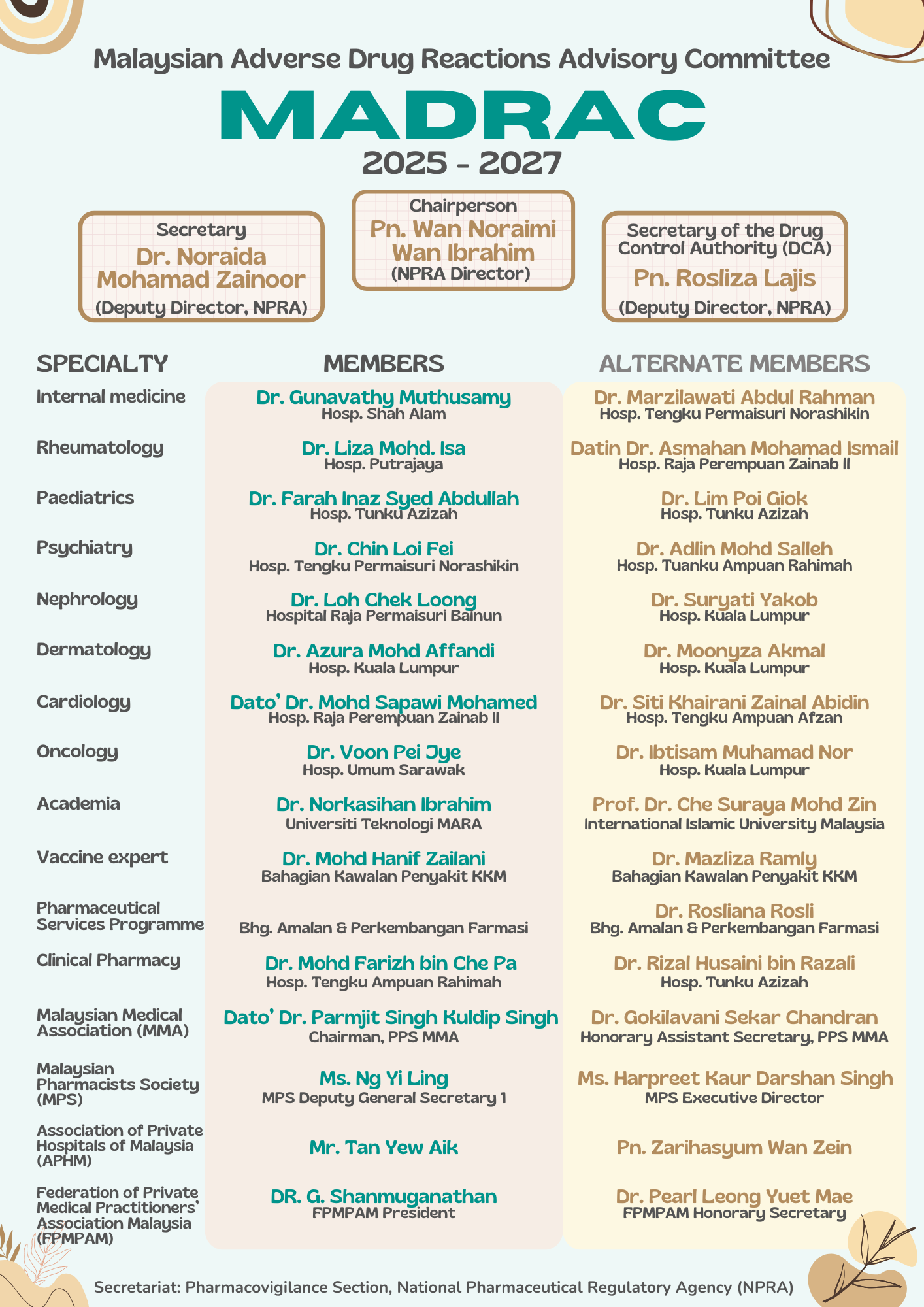
The members of MADRAC (Malaysian Adverse Drug Reactions Advisory Committee) are selected based on their qualifications and expertise with regard to their respective fields of practice. They serve on the Committee for a renewable period of three years.
MADRAC Members Session 2025 - 2027
NOMINATION CRITERIA FOR THE MADRAC MEMBERS
1. MADRAC shall consist of the following members:
1.1 Three (3) members in their ex-officio capacity:
a) Director of National Pharmaceutical Regulatory Agency (NPRA) - Chairperson
b) Secretary of DCA, NPRA
c) Deputy Director Centre for Compliance and Quality Control, NPRA - Secretary
1.2 Sixteen (16) members appointed by the Director General of Health.
2. Criteria for nominating the MADRAC members are as follows:
2.1 Consultant Specialists/ Senior Consultant Specialists from MOH nominated by the respective heads of services/departments:
i. 2 consultant physicians
ii. 1 consultant dermatologist
iii. 1 consultant rheumatologist
iv. 1 consultant psychiatrist
v. 1 consultant nephrologist
vi. 1 consultant pediatrician
vii. 1 consultant oncologist
viii. 1 consultant with expertise in vaccines
ix. 1 pharmacist from the Pharmaceutical Services Programme
x. 1 pharmacist from a public health facility (hospital/health clinic)
a) Must have at least 2 years of service with MOH remaining;
b) Not among the members/alternate members of DCA
2.2 A pharmacist from a Public Institution of Higher Learning, and nominations will be requested from universities with teaching hospitals
a) Must have at least 2 years of service with the Ministry of Higher Education remaining;
b) Not among the members/alternate members of DCA
2.3 Representative from the private healthcare sector
i. 1 member of the Malaysian Pharmaceutical Society (MPS)
ii. 1 member of the Association of Private Hospitals Malaysia (APHM)
iii. 1 member from the Malaysian Medical Association (MMA)
iv. 1 member from the Federation of Private Medical Practitioner’s Association, Malaysia (FPMPAM)
a) Must possess relevant professional qualifications (doctor or pharmacist) and experience;
b) Must have retired from the Public Service
3. Alternate members:
In respect of appointed members, an alternate member shall be similarly qualified as the substantive member and appointed by the Director General of Health, Malaysia.
4. All members of MADRAC are appointed by the Director General of Health for a term of 3 years.
If you have any queries regarding the appointment of MADRAC members, please contact the NPRA Pharmacovigilance Section at email: This email address is being protected from spambots. You need JavaScript enabled to view it.
Last updated: 30 Apr 2025











