i) Name of Active Ingredient:
· Please refer Appendix 8.1 List of Prohibited and Restricted Active Ingredients and Combinations
· Please select active ingredient from the search database by clicking button ‘click here to search’. If an active ingredient is not listed in the database, please click button ‘Not Listed Ingredient’. Automatic e-mail will be send to NPRA for verification. Please ensure that the spelling is accurate.
· The actual raw material that is employed in the manufacturing process shall be named. For example:
- Where the raw material used is the salt (e.g. ampicillin trihydrate) which will yield an equivalent effective component from its base content (i.e. ampicillin), the substance name is the salt and the equivalent base component should be indicated in remarks on substance (if any) field. ***
- Similarly where a chemical is a component in the ingredient (e.g. iron in ferrous sulfate; or EPA and DHA in fish oil), the component details shall be stated in the remarks field if a label claim of the component is made for the product, and the actual raw material used declared as the active ingredient.
· International Non-proprietary Names (INN), approved names, pharmacopoeia names of ingredients shall be used whenever possible.
· After each ingredient entry is correctly made, click the button ‘add/ save’. The button ‘remove’ will allow for corrections to an entry under this heading. To remove item, please select item from the listing and click ‘remove’.
ii) Strength of active ingredient:
· Please enter strength of active ingredient (numerical) and then select unit weights and measures from the drop-down list.
· Content of ingredients shall be expressed as appropriate in the following manner :
a. quantity per dose unit
(e.g. for unit dose formulations - tablet, capsule, lozenge, etc.)
b. percentage composition - %w/w, %w/v, %v/v, etc.
(e.g. for products without defined dose unit such as ointments, creams, solutions, etc.)
c. weight per ml.
(e.g. for solutions, injections, etc.)
d. quantity (percentage or amount) per measured dose
(e.g. oral liquids, metered aerosols, drops, etc.)
· Metric weights and measures shall be used.
· In cases where product contains active ingredient(s) that cannot be definitely identified (e.g. certain biological products) state the name of the material to which activity is ascribed and, where appropriate, the potency or activity of the product.
iii) Remarks on active ingredient (if any):***
· This field shall be used where the raw material in product formulation yields an equivalent active component.
After each ingredient entry is correctly made, click the ‘add/ save’ button. The remove button will allow for corrections to an entry under this heading. To remove item, select item from the listing and click remove.
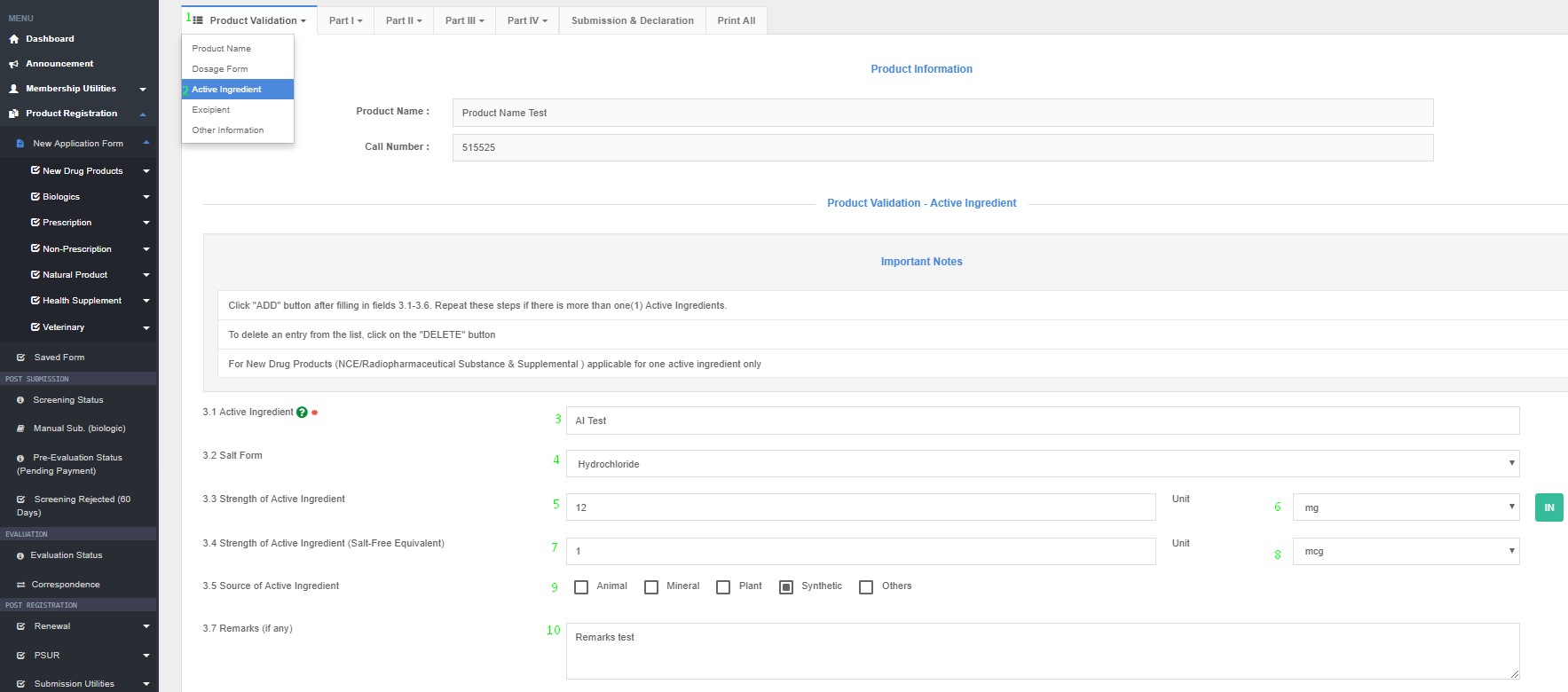
How to Access Active Ingredient in QUEST3+ System ?
Product Registration >> New Application Form >> Product Validation >> Active Ingredients