Further information related to this safety issue has become available. Readers are advised to also refer to the NPRA Safety Alert on Valproate (Sodium Valproate, Valproic Acid): Potential Risks to Offspring Following Paternal Exposure, and Male Infertility published on 19 August 2025.
Background of Safety Issue
Sodium valproate has been known to cause congenital malformations in neonates (approximately 10% of cases), and serious developmental problems among children in up to 30-40% of cases whose mothers were exposed to sodium valproate during pregnancy.
Examples of congenital malformations include neural tube defects, facial dysmorphism, cleft lip and palate, and multiple anomalies involving various body systems. Available data show that children with a history of sodium valproate exposure in utero may experience delays in their early development and had increased risk of developmental disorders compared to the general study population.
The European Medicines Agency (EMA) restricted sodium valproate use in pregnancy among epileptic, migraine, and bipolar disorder patients. EMA also instructed product registration holders of sodium valproate products to prepare additional educational materials.
Considering the severity of this risk, the Drug Control Authority (DCA) of Malaysia has taken similar measures to contraindicate the use of sodium valproate in bipolar disorder and epilepsy. However, sodium valproate can still be prescribed for epileptic women only if there is no suitable alternative medicine available to treat the patient and the conditions of the pregnancy prevention programme* are met.
Additional steps taken by the DCA include requiring product registration holders to prepare and supply several education materials for sodium valproate, as follows:
- Patient Card
- Annual Risk Acknowledgement Form (for prescribers)
- Guide for Healthcare Professionals
- Guide for Female Patients/ Caregivers
Adverse Drug Reaction Reports
NPRA has received 437 reports with 766 adverse events suspected to be related to sodium valproate use. Two (2) of these reports were associated with use during pregnancy, with congenital malformations reported including spina bifida, meningomyelocele, exomphalos and congenital diaphragmatic hernia.
Adverse drug reactions data from the World Health Organisation (WHO) database revealed 2,813 reports involving sodium valproate use were linked to congenital, familial and genetic disorders, such as spina bifida (413), dysmorphism (399) and congenital anomaly (332).
Advice for Healthcare Professionals
1. Sodium valproate is contraindicated in the following situations:
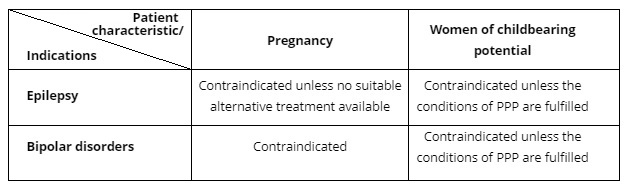
Abbreviation: PPP = Pregnancy Prevention Programme*
2. All female patients who are considering sodium valproate therapy must be informed on the following points:
- There is a risk of congenital malformation and neurodevelopmental problems in children whose mothers were exposed to sodium valproate during pregnancy.
- Pregnancy testing is required before starting sodium valproate and throughout treatment, as necessary.
- The use of effective contraception during the entire duration of treatment is important.
- Consult a doctor if they are planning for pregnancy.
- Continue taking sodium valproate even when pregnancy is suspected or already pregnant, and immediately see their doctor.
- Ensure they have received educational materials (e.g. patient card and patient information leaflet).
3. If sodium valproate is required for the patient and other treatment options are ineffective or not tolerated, please ensure the following:
- Treatment should only be initiated after pregnancy has been excluded (negative pregnancy test).
- Sodium valproate should be prescribed as monotherapy and at the lowest effective dose. A prolonged release formulation is recommended to avoid high peak plasma concentrations and the daily dose should be divided into at least two single doses.
- Annual review should be carried out, and ad-hoc treatment review conducted when required. The benefit and risk should be carefully reconsidered during every treatment review.
- When patient is planning for pregnancy, all efforts should be made to switch to appropriate alternative treatment prior to conception, if possible.
- In the case where sodium valproate must be used during pregnancy, prenatal monitoring is recommended to detect any malformations.
A new directive [Ruj. Kami: (21) dlm. BPFK/PPP/07/25 Jld. 3] has been issued by NPRA for new updates to product label, product package insert and consumer medication information leaflet, thereby replacing the previous directive [Bil.(3) dlm. BPFK/PPP/07/25 Jld. 1] dated 11 October 2016.
*For a full description of the product information update and the Pregnancy Prevention Programme, please refer to the new directive.
References:
- Meador K., Reynolds M.W., Crean S, Fahrbach K, Probst C (2008). Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 81(1):1-13.
- Weston J., Bromley R., Jackson C.F., Adab N., Clayton-Smith J., Greenhalgh J., Hounsome J., McKay A.J., Tudur Smith C., Marson A.G. (2016). Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database of Systematic Reviews, Issue 11. Art. No.: CD010224
- Bromley R.L., Mawer G, Love J, Kelly J, Purdy L, McEwan L et al. (2010). Early cognitive development in children born to women with epilepsy: a prospective report. Epilepsia; 51(10):2058-65.
- Cummings et al. (2011). Neurodevelopment of children exposed in utero to lamotrigine, sodium valproate and carbamazepine. Arch. Dis. Child; 96: 643-647
- Meador K. et al. (2009). Cognitive Function at 3 years of age after fetal exposure to antiepileptic drugs. NEJM; 360 (16): 1597- 1605
- Thomas S.V. et al. (2008). Motor and mental development of infants exposed to antiepileptic drugs in utero. Epilepsy and Behaviour; (13):229-236
- Christensen J. et al. (2013). Prenatal Sodium valproate Exposure and Risk of Autism Spectrum Disorders and Childhood Autism. JAMA; 309(16):1696-1703
- Cohen M.J. et al. (2011). Fetal Antiepileptic Drug Exposure: Motor, Adaptive and Emotional/Behavioural Functioning at age 3 years. Epilepsy Behav.; 22(2):240-246
- European Medicine Agency (2019). New product information wording – Extracts from PRAC recommendations on signals. EMA/PRAC/826450/2018.
- Malaysian Adverse Drug Reaction Database, NPRA. [Accessed on September 2019]
- WHO Vigilyze database, Uppsala Monitoring Centre. [Accessed on September 2019]
DISCLAIMER
This publication is aimed at health professionals. The information is meant to provide updates on medication safety issues, and not as a substitute for clinical judgement. While reasonable care has been taken to verify the accuracy of the information at the time of publication, the NPRA shall not be held liable for any loss whatsoever arising from the use of or reliance on this publication.










