|
1.2 |
||
|
1.2.1 |
||
|
1.2.2 |
||
|
1.2.3 |
||
|
1.2.4 |
||
| 1.2.5 | General Classification Flowchart Of Food-Drug Interphase (FDI) Under Food Or Drug | |
| 1.2.6 | Additional Notes | |
| 1.2.7 | Medical Device - Drug - Cosmetic Interphase Products | |
1.2 FOOD - DRUG INTERPHASE PRODUCTS
This guide serves to assist in determining if a product is to be regulated by the National Pharmaceutical Regulatory Division (NPRA) or by the Food Safety and Quality Division (FSQD) of the Ministry of Health Malaysia.
1.2.1 INTRODUCTION
Malaysians are now more health conscious and there is generally greater awareness of the importance of nutrition to overall well-being. In recent years, many consumers also rely on a variety of “dietary supplements” to improve their health. These diverse products are freely available through a myriad of outlets. A variety of products are available in the market, supposedly for the maintenance, prevention and even treatment of chronic diseases. These products may range from foods modified to have special properties or pure forms of vitamins and minerals to extract of various botanical or animal products.
It is important to monitor and regulate the marketing and sale of these products so as to protect the interest and health of the consumer. Some of these products are not clearly defined as “food” or “drugs” but are freely marketed. Such products include a variety of so-called health products and have been termed as “food-drug interphase (FDI) products”.
In order to better define and regulate the FDI products, both the NPRA and the FSQD, Ministry of Health Malaysia formed the Committee for the Classification of Food-Drug Interphase Products in 2000. The main Terms of Reference of the Committee is to assist both Divisions in classifying, in a consistent manner, an application from the industry which is not clearly defined either as a food or drug product. The Committee also serves as a platform in strengthening and updating the relevant regulations as well as to provide scientific input on these products.
CHARGES FOR PRODUCT CLASSIFICATION
|
Processing fee |
Timeline |
|
RM 300 per product for each application |
7-14 working days upon receipt of complete and satisfactory application |
1.2.2 DEFINITION OF FDI PRODUCTS
Generally FDI products are products with combination of food ingredients and active ingredients for oral consumption. Examples of food ingredients are fruit, vegetables, meat, poultry, milk, cocoa and cereal. Examples of active ingredients are vitamins, minerals, herbs, enzymes, probiotics, prebiotics, amino acids, peptides, coral calcium, fatty acids, collagen, chia seed, astaxanthin, lutein and other ingredients that are not traditionally consumed as food. FDI products may be presented in the form of powder, liquid, semisolid forms such as gel/jelly, chewable tablet, drops, granule etc.
Such products as below are not categorized as FDI products due to its presentation and function:
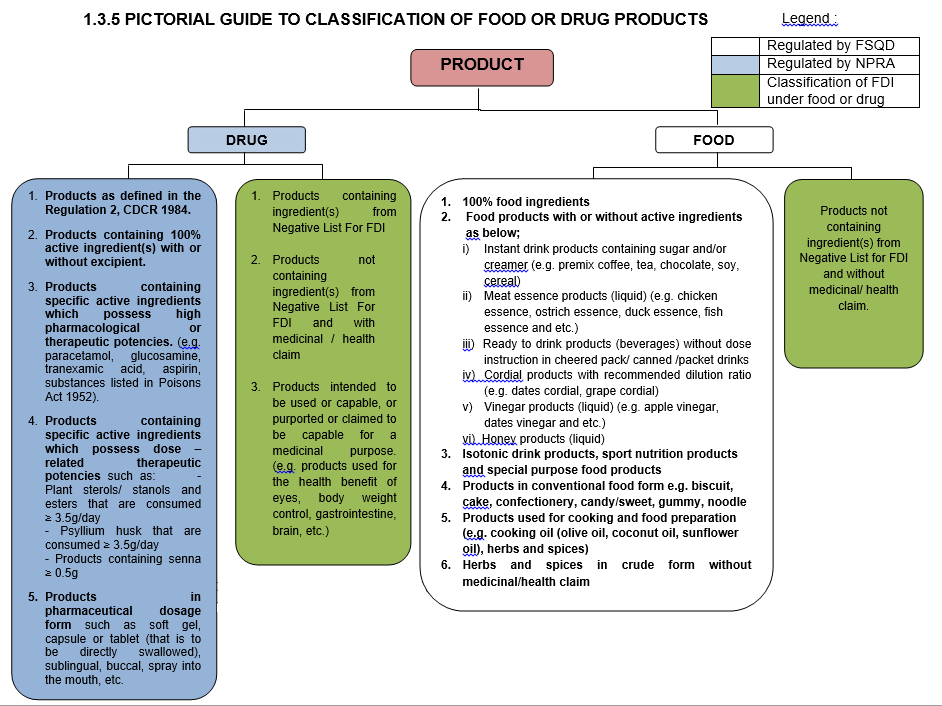
A. FOOD PRODUCTS THAT ARE NOT CATEGORIZED AS FDI PRODUCTS AND REGULATED BY FSQD INCLUDE :
1. 100% food ingredients.
2. Food products with or without active ingredients ( eg; herbs, vitamins, minerals, etc) as below:
i) Instant drink products containing sugar and creamer (e.g. premix coffee, tea, chocolate, soy, cereal).
ii) Meat essence products (liquid) (e.g. chicken essence, ostrich essence, duck essence, fish essence etc.)
iii) Ready to drink products (beverages) without dosing instruction in cheered pack/ canned / packet drinks.
iv) Cordial products with recommended dilution ratio (e.g. dates cordial, grape cordial)
v) Vinegar products (liquid) (e.g. apple vinegar, dates vinegar etc.)
vi) Honey products (liquid).
3. Isotonic drink products, sport nutrition products and special purpose food products.
4. Products in conventional food form e.g. biscuit, cake, confectionery, candy/sweet, gummy, noodle.
5. Products used for cooking and food preparation (e.g. cooking oil (olive oil, coconut oil, sunflower oil), herbs and spices).
6. Herbs and spices in crude form without medicinal/health claim.
B. PRODUCTS THAT ARE NOT CATEGORIZED AS FDI PRODUCTS AND REGULATED BY NPRA INCLUDE :
1. Products containing active ingredient(s) with or without excipient ; or
2. Products containing specific active ingredients which possess high pharmacological or therapeutic potencies. Examples of the ingredients are paracetamol, glucosamine, tranexamic acid, aspirin, substances listed in Poisons Act 1952 ; or
3. Products containing specific active ingredients which possess dose-related therapeutic potencies such as:
i) Plant sterols/ stanols and esters that are consumed ≥ 3.5g/day
ii) Psyllium husk that are consumed ≥ 3.5g/day
iii) Products containing senna ≥ 0.5g ; or
4. Products in pharmaceutical dosage form such as soft gel, capsule or tablet (that is to be directly swallowed), sublingual, buccal, spray into the mouth, etc.
1.2.3 CLASSIFICATION FOR FDI PRODUCTS
It is important to determine the category of a product that falls within the food-drug interphase (FDI) whether the products are regulated as drug (under the NPRA’s purview) or, as food (under the FSQ’s purview) because different regulatory requirements apply.
The classification of FDI products are based on criteria, as outlined below:
a) Main criteria
i) Negative List For FDI as listed in Table 1: Negative List For FD
- FDI products containing ingredient(s) from Negative List for FDI shall be regulated by NPRA ; or
ii) Medicinal/ health claim refer to the term “medicinal purpose” as stipulated in the Sales of Drug Act 1952, Section 2:
- FDI products not containing ingredient(s) from Negative List For FDI and with medicinal/ health claim shall be regulated by NPRA ; or
- FDI products not containing ingredient(s) from Negative List For FDI and without medicinal/ health claim shall be regulated by FSQD.
iii) Products intended to be used or capable, or purported or claimed to be capable for a medicinal purpose (e.g. products used for the health benefit of eyes, body weight control, gastrointestine, brain, etc.) shall be regulated by NPRA.
b) Other criteria
- When there is greater uncertainty regarding the safety of a FDI product, such shall be regulated by NPRA. This is to enable closer monitoring of such products, so as to safeguard the health of the consumer.
Reference : Pekeliling Kriteria Baru Pengkelasan Produk (07 August 2014)
1.2.4 NEGATIVE LIST FOR FDI
Table 1: Negative List For FDI
| No. | Ingredient | Common/Other name |
| 1 | Actaea racemosa | Black Cohosh, Cimicifuga racemosa |
| 2 | Antiaris toxicaria (Pers.) Lesch. | Bark cloth tree, antiaris, false iroko, false mvule, upas tree |
| 3 | Artemisia Spp. (all species) | Wormwood, Mugwort |
| 4 | Aspidosperma Quebracho-Blanco Schltdl | Kebrako, White Quebracho |
| 5 | Atropa Spp. (all species) | Antropa belladonna (deadly nightshade) |
| 6 | Azadirachta indica | Nimba, Neem |
| 7 | Bile | |
| 8 | Brucea javanica, Brucea amarissima | Sumatrana amarissimus, Java brucea |
| 9 | Bufo gargarizans Cantor, Bufo melanostictus Schneider, Bufo vulgaris Lour | Toad, Samsu, kodok, kerok |
| 10 | Calotropis Spp. (all species) | Apple of Sodom, Crown flower |
| 11 | Cannabis Spp. (all species) | Marijuana, Hemp |
| 12 | Catharanthus Spp. (all species) | Periwinkle |
| 13 | Chelidonium majus | Celandine, Great Celandine, Nipplewort |
| 14 | Chondodendron Spp. (all species) | |
| 15 | Claviceps Spp. (all species) | Ergot |
| 16 | Colchicum Spp. (all species) | Autumn crocus, Meadow saffron, Naked lady |
| 17 | Conium maculatum | Hemlock |
| 18 | Coptis chinensis, Coptis teeta | Chinese Goldthread |
| 19 | Croton tiglium L. | Croton |
| 20 | Datura spp. (all species) | Jimson weed, Devil’s apple, Green Dragon, Zombie’s Cucumber, Moon Weed, Trumpet Lily, Stinkweed |
| 21 | Digitalis spp.(all species) | |
| 22 | Dioscorea Hispida | |
| 23 | Dryobalanops lanceolata Burck | Borneo camphor, Kapur, Malay Camphor, Sumatra camphor |
| 24 | Dryopteris Spp. (all species) | Mountain woodfern, Spinulose woodfern, Spreading woodfern, Fancy fern |
| 25 | Euphorbia Spp. (all species) | Spurge |
| 26 | Fritillaria spp. | Fritillary Bulb |
| 27 | Gamma-amino Butyric Acid (GABA) | |
| 28 | Garcinia Morella Desr. | Gamboge |
| 29 | Gelsemium semperi virens | Palaung Thay |
| 30 | Glucosamine | |
| 31 | Glutathione | |
| 32 | Gypsum Fibrosum | |
| 33 | Hyaluronic acid | |
| 34 | Hyoscyamus Spp. (all species) | |
| 35 | Hypericum perforatum | St. John’s Wort |
| 36 | Juniperus sabina | Savin, Savine |
| 37 | Mahonia aquifolium, Mahonia repens, Mahonia nervosa | Mahonia Aquifolium: Oregon Grape, Mountain Grape, Barberry. Mahonia Repens: Creeping Barberry, Creeping Mahonia, Creeping Oregon-Grape |
| 38 | Melanorrhoea usitata Wall. | Vanish tree |
| 39 | Monascus purpureus | Red yeast rice |
| 40 | Mucuna pruriens | Cowhage, Cowage |
| 41 | Mylabris phalerata, Mylabris cichorii | Blister beatle, Mylabris |
| 42 | Natto extract | Fermented soy bean extract |
| 43 | Nerium indicum | Indian oleander, Exile Tree. |
| 44 | Nerium oleander | Indian oleander, Exile Tree. |
| 45 | Pearl | |
| 46 | Phellodendron amurense, Phellodendron chinense | Amur Cork tree |
| 47 | Placenta | |
| 48 | Plumbago indica | Rose-coloured leadwort |
| 49 | Plumbago zeylanica | White leadwort |
| 50 | Psilocybe cubensis | Boomers, Gold caps |
| 51 | Rauvolfia Spp. (all species) | |
| 52 | Resveratrol | |
| 53 | Sanguinaria canadensis | Bloodroot, Indian Paint |
| 54 | Scilla sinensis | |
| 55 | Simmondsia Chinesis | Jojoba |
| 56 | Sophora tomentosa | Sea coast Laburnum, Silver Bush |
| 57 | Spigelia marilandica | Worm grass, Pinkroot |
| 58 | Stichopus spp. | Gamat |
| 59 | Strophanthus spp.(all species) | Kombe |
| 60 | Strychnos ignatii, Strychnos lucida, Strychnos roberans | Nux-vomica |
| 61 | Symphytum peregrinum | Comfrey |
Notes:
This list :
· is a compilation by the FDI committee.
· is not meant to be exhaustive and will be reviewed from time to time.
· shall be read in conjunction with the current laws and regulations together with other relevant legislations, where applicable, governing pharmaceutical and natural products for human use in Malaysia
Notes:
Applicant may verify on FDI product classification with NPRA in order to determine whether the product shall be registered by the Authority or otherwise by seeking classification service from NPRA
| Document Name | MS Word | Updated | |
|---|---|---|---|
| NPRA 300.1: Borang Pengkelasan Produk (digunakan mulai 1 Jan 2016) | Download | Download |
Reference Circular:Bil.(97)dlm.BPFK/PPP/01/03 Jld. 2
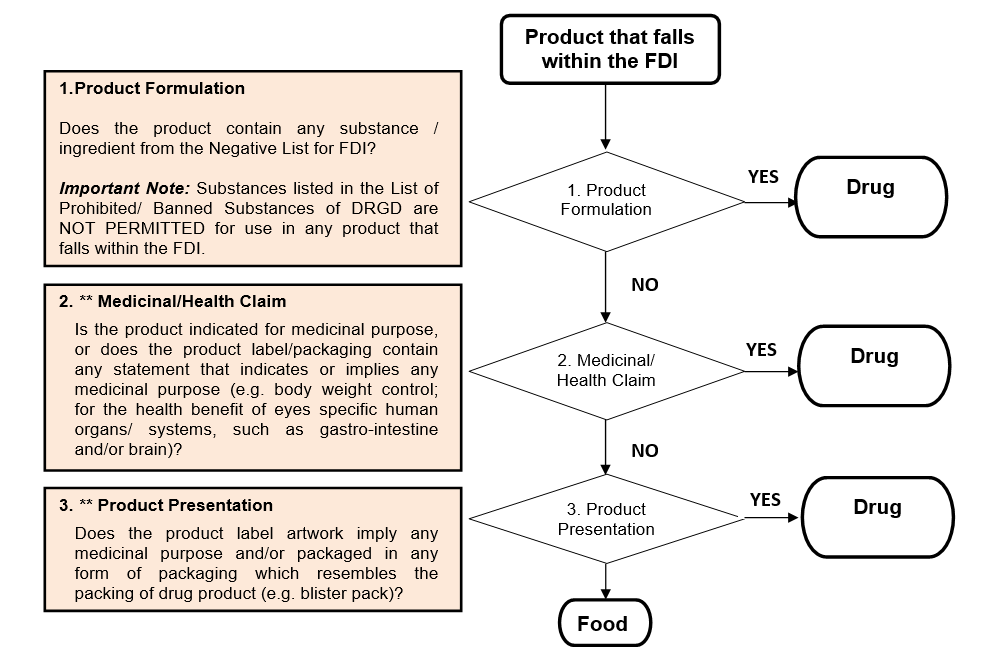
1.2.5 GENERAL CLASSIFICATION FLOWCHART OF FOOD-DRUG INTERPHASE (FDI) UNDER FOOD OR DRUG
-
It is important to determine the category of a product that falls within the food-drug interphase (FDI) whether the products are regulated as drug (health supplement or natural product under the NPRA’s purview) or, as food (under the FSQ’s purview) because different regulatory requirements apply. Therefore, the following flowchart serves only as guide to help you determine the category of the product that falls within the FDI.
-
Should you have any doubt or uncertainty pertaining to the category of your product, you may contact the relevant regulatory agencies for clarification, or seek classification service from the NPRA by submitting a classification application.
-
Please take note that you are encouraged to familiarize yourself with the governing legislations and other regulatory requirements and guidelines that apply to your product before using this guide.
Note: ** NPRA reserves the right to use its discretion to make decision if issue of subjectivity arises.
1.2.6 ADDITIONAL NOTES
-
Substances listed in the prohibited/ banned ingredient list of the Drug Registration Guidance Document (DRGD) and Schedule Poison shall not be permitted for use in any FDI products.
-
Products categorized as a natural product are not allowed to contain creamer.
-
Food products are not allowed to be packed in blister pack/ any other form of packaging which resembles the packing of drug product.
-
Any foods or combination of foods that are regulated by FSQD shall not be in pharmaceutical dosage form, such products are advised to reformulate into a non-pharmaceutical dosage form.
-
Products containing only ingredient(s) such as roselle, jasmine, rose, chamomile, chrysanthemum flower, ginger (rhizome), vanilla(stem), mint leaf, lemon peel and cinnamon bark (with/without Camelia sinensis) will be regulated by FSQD.
-
Fruit ingredients that are not commonly consumed as food in Malaysia will be considered as active ingredient.
1.2.7 MEDICAL DEVICE - DRUG - COSMETIC INTERPHASE PRODUCTS
INTRODUCTION
Medical Device-Drug-Cosmetic Interphase (MDDCI) Products are those products that are not clearly defined as a medical device or drug/cosmetic in accordance to the Medical Device Act 737, Control of Drugs and Cosmetics Regulations 1984 and Sale of Drugs Act 1952.
Registration of drug products/ notification of cosmetics that has been classified must follow the requirements that have been set forth as follows:
- Drugs & Cosmetics – The registration/ notification regulated by the NPRA is in accordance with the requirements set forth in the Poisons Act 1952 and its Regulations, Sales of Drugs Act 1952 and the Control of Drugs and Cosmetics Regulations 1984;
- Medical Device – The registration regulated by Medical Device Authority is in accordance with the requirements set forth in the Medical Devices Act 2012 (Act 737).
Combination products includes:
- i) A product comprised of two or more regulated components, i.e., drug/device, biological/device, or drug/device/biological, that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
- ii) Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products.
For Interphase Product and Combination Product (Device-Drug or Drug-Device), it will be regulated according to the classification that has been made and by the relevant agencies.
Please refer ;
- Directive No. 4 Year 2017, Ref. (9) dlm.BPFK/PPP/07/25 Jld. 1 : DIREKTIF KUATKUASA PEMAKAIAN GUIDELINE FOR REGISTRATION OF DRUG-MEDICAL DEVICE AND MEDICAL DEVICE-DRUG COMBINATION PRODUCTS
- Guideline For Registration Of Drug-Medical Device and Medical Device-Drug Combination Products
1.4.2 CLASSIFICATION CRITERIA
The following may be used as criteria to assist in the classification of products:
a)The primary intended purpose of the product;
b)The primary mode of action/ the principal mechanism of action by which the claimed effect or purpose of the product is achieved;
- Drug is based on pharmacological, immunological or metabolic action in/on the body; but
- Medical device does not achieve its primary mode of action in or on the human body by pharmacological, immunological or metabolic means, but may be assisted in its intended function by such means.;
c)Active ingredient, indication and pharmaceutical dosage form (these are the main criteria for classification of the drugs);
d)Classification of the products in reference countries.
For classification of MDDCI products and combination products as decided by the committee, please refer to Table III. It shall be used as guidance for classification only.
Applicant shall verify on MDDCI product classification with NPRA in order to determine whether the product shall be registered by the Authority or otherwise.
Table III: SUMMARY OF MEDICAL DEVICE-DRUG-COSMETIC INTERPHASE (MDDCI) PRODUCT CLASSIFICATION DECISION
|
NO |
PRODUCT |
INTENDED PURPOSE/ INDICATION AND MODE OF ACTION (MOA) |
CATEGORY |
CUSTODIAN DIVISION |
|
|
1. |
Aqueous Cream Product |
As an emollient cream with moisturizing properties to promote healing and relief to the symptoms of skin dryness, impaired barrier function, skin problems/ diseases. |
OTC DRUG |
NPRA |
|
|
2. |
Blood bag containing anticoagulant/ preservation agent |
To collect and preserve blood and its components (for use with cytapheresis device only)
NOTE : It is not for direct intravenous infusion. |
MEDICAL DEVICE |
MDA |
|
|
3. |
Catheter Lock/ Flush Solutions (eg. heparinised saline, sodium citrate solution) |
As an anticoagulant for use as a catheter lock / flush solution for flushing off catheters and cannulas to maintain catheter/ cannula patency and to prevent coagulation of blood or infection in the cathether.
NOTE : - It is not indicated for therapeutic use. - Contraindicated for direct systemic administration. |
MEDICAL DEVICE
|
MDA |
|
|
4. |
Collagen Hemostatic Agents (fibrillar or soft, pliable pad/sponge or loose fibres) |
A sterile, bioabsorbable device derived from animal collagen (e.g., bovine or porcine collagen) designed to produce a rapid haemostasis through platelet activation/aggregation (which initiates the haemostatic cascade leading to a fibrin clot) during a surgical procedure. It is applied directly to the wound where it remains to be absorbed by the body; it is not dedicated to a specific anatomy/application and does not contain an antimicrobial agent
|
MEDICAL DEVICE |
MDA |
|
|
5. |
Dental Products |
||||
|
i. Fluoride dental preparations (eg. toothpaste, tooth powder, mouthwash, dental varnish) |
a. To maintain oral hygiene. |
COSMETIC (If concentration of fluoride ≤1500ppm) |
NPRA |
||
|
b. To maintain oral hygiene and prevent oral diseases. |
DRUG (If concentration of fluoride is >1500ppm) |
NPRA |
|||
|
c. A liquid substance used for the protection of pulpal tissue and to provide a marginal seal to newly placed amalgam restorations. A thin coating of this solution is applied over the tooth’s surfaces before placement of restorations. It is used as a protective agent for the tooth against constituents of restorative materials. After application, this device cannot be reused. |
MEDICAL DEVICE |
MDA
|
|||
|
d. As a desensitizing agent for the treatment of hypersensitive teeth, for sealing the dentinal tubules for cavity preparations or on sensitive root surfaces or to line cavity preparations under amalgam restorations. |
MEDICAL DEVICE |
MDA |
|||
|
ii. Root canal filling incorporating antibiotic
|
To seal the canal and disinfect the dentinal walls by diffusing through dentine. The antibiotic provides ancillary actions as bactericidal antibiotic and anti-inflammatory agent to assist in reducing pain and in maintaining a bacteria-free environment within the root canal. |
Device-Drug combination product regulated as MEDICAL DEVICE |
MDA |
||
|
6. |
Dialysis Products |
||||
|
It is used for the exchange of solutes across the peritoneum of the patient (in this case, used as a semi-permeable membrane) |
DRUG
|
NPRA |
||
|
It is used for the exchange of solutes with blood through a system of extracorporeal filters. |
DRUG |
NPRA |
||
|
It is used for the exchange of solutes with blood through a semi-permeable membrane in the dialyser of a haemodialysis system. |
MEDICAL DEVICE |
MDA |
||
|
It is used as a replacement solution in haemodiafiltration. NOTE : Haemodiafiltration is the combination of haemodialysis and haemofiltration performed either simultaneously or sequentially. |
DRUG |
NPRA |
||
|
7. |
Drug-Eluting Beads (Produced from biocompatible polyvinyl alcohol hydrogel modified with sulphonate groups in phosphate buffered saline.)
|
It is an embolic agent which is intended to be loaded with a chemotherapy agent, eg. doxorubicin for the purpose of treatment of malignant hypervascularised tumour(s) by embolisation of vessels and occlusion of blood flow supplying malignant hypervascularised tumour(s) and as a secondary action, delivers/elutes a local, controlled, sustained dose of the chemotherapy agent directly to the tumour(s). |
If the beads are sold separately from the drug, it will be classified as MEDICAL DEVICE
If the beads and drug are packaged and sold together, it will be classified as Drug-device combination product regulated as DRUG |
MDA/NPRA |
|
|
8. |
Drug-Eluting Stents (DES) |
For use in angioplasty or coronary stenting procedures. |
Device-Drug combination product regulated as MEDICAL DEVICE |
MDA |
|
|
9. |
Drug - Delivery Products Regulated as Drug Products (eg. insulin prefilled pen/ syringes, asthma inhalers, intrauterine contraceptives whose primary purpose is to release progestogens)
|
To administer pharmacologically active substance |
Drug-device combination product regulated as DRUG
NOTE: The device component will be regulated on a case to case basis.
|
NPRA |
|
|
10. |
Enteral Feeding Kit (containing Iodine Pack drug) |
A collection of sterile devices that includes tubing and other materials intended to administer nutrient liquids directly into the stomach, duodenum, or jejunum of a patient by means of gravity or an enteral pump. |
Device-Drug combination product regulated as MEDICAL DEVICE |
MDA |
|
|
11. |
Eye Products |
||||
|
A sterile substance used to provide supplemental lubrication/hydration/ moisturization to the eyes to treat/ alleviate symptoms of soreness, burning, irritation and discomfort caused by dry, tired, and/or strained eyes resulting from dry eye syndrome, ageing/ hormone changes (menopause), or environmental factors (e.g., pollution, dust, heat, smoke and air conditioning). |
MEDICAL DEVICE (If it contains an active substance with pharmacological, immunological or metabolic primary mode of action, it will be classified as DRUG) |
MDA |
||
|
It is used to assist in performing ophthalmic surgery, e.g., to maintain the shape of the eyeball during the intervention, preserve tissue integrity, protect from surgical trauma, or to function as a tamponade during retinal reattachment. |
MEDICAL DEVICE |
MDA |
||
|
To reduce fatigue from work stress or lack of sleep. |
MEDICAL DEVICE |
MDA |
||
|
12. |
General Purpose Surgical or Barrier Drapes (A sterile protective covering made of natural or synthetic materials, or both.) |
To isolate a site of surgical incision or a surgical field from contamination (e.g., microbial, substance) in various clinical settings (e.g., in an operating room or catheterization laboratory). The device may also be used to protect a patient from heat/flame during a surgical procedure. This is a reusable or single use device. |
MEDICAL DEVICE (If it incorporates an ancillary pharmacologically active substance, it will be classified as Device-Drug combination product regulated as MEDICAL DEVICE) |
MDA |
|
|
13. |
General-body orifice lubricant
|
Lubricant intended to facilitate entry of a diagnostic or therapeutic device into a body orifice by reducing friction between the device and the body; Lubricant during catherisation, probing,endoscopy, changing fistula catheters,intubation,and prevention of iatrogenic injuries to the rectum and colon. E.g ancillary local anaesthetic: lidocaine |
MEDICAL DEVICE
(If it incorporates an ancillary pharmacologically active substance, it will be classified as Device-Drug combination product regulated as MEDICAL DEVICE |
MDA
|
|
|
14. |
Heat Pad/ Cooling Pad |
To relief aches and pains. |
MEDICAL DEVICE |
MDA |
|
|
15. |
In vivo diagnostic agents |
a. For diagnostic purposes, eg. : - X-ray / MRI contrast media - NMR enhancing agents - Opthalmic diagnostic agents, eg. staining agent such as fluorescent ophthalmic strips for diagnostic purposes - Carrier solutions to stabilize microbubbles for ultrasound imaging - Radiopharmaceuticals for diagnostic use eg 14C- Urea Capsule for H pylori test |
DRUG
|
NPRA |
|
|
b. As Diagnostic Test Kit consist of drug and analyser
|
DRUG-DEVICE combination product regulated as DRUG NOTE: The device component will be regulated on a case to case basis. |
NPRA
|
|||
|
c. As diagnostic analyser only (without drug) |
MEDICAL DEVICE |
MDA |
|||
|
16. |
Irrigation solutions
|
For mechanical cleansing and rinsing including those used in the eye such as for cleansing of the eye, body tissues, body cavities, wounds or irrigation of a special tube called a catheter which is used to drain the bladder.
|
MEDICAL DEVICE
(If it contains a pharmacologically active substance, it will be classified as DRUG) |
MDA |
|
|
17.
|
Medical gases
|
|
DRUG
|
NPRA |
|
|
DRUG
|
NPRA |
|||
|
18. |
Medicinal Patch
|
To relieve fatigue, body aches, joint pains; To regulate hormone imbalance |
DRUG
|
NPRA |
|
|
19. |
Nail Anti-fungal Products (eg. pen applicator containing acetic acid/ lactic acid)
|
Treatment of onychomycosis (fungal nail infection) by lowering the pH of the nail bed, thus creating a micro-environment that is hostile to fungal growth.
|
MEDICAL DEVICE |
MDA |
|
|
20. |
Nasal inhaler
|
A hand-held device designed to administer substances directly into the nares of a patient, to serve as a barrier against external influences by formation of a moisturizing film on the nasal mucosa. |
MEDICAL DEVICE |
MDA |
|
|
21. |
Oral care products |
||||
|
Artificial Saliva / Saliva Substitute/ Replacement
|
Solutions used to mimic and replace/substitute normal saliva in the symptomatic treatment of dry mouth (xerostomia). Generally contain viscosity-increasing agents, such as mucins or cellulose derivatives such as carmellose as well as electrolytes, including fluoride. They seldom relieve symptoms for more than 1 or 2 hours and does not stimulate saliva production. |
MEDICAL DEVICE
|
MDA
|
||
|
22. |
Other topical antiseptics/ disinfectants |
||||
|
For use on human skin and intended to be used for a medical purpose, eg pre/post injection, wound cleaning etc. |
DRUG |
NPRA |
||
|
Intended for the disinfection of medical devices. |
MEDICAL DEVICE |
MDA |
||
|
23. |
Peeling/Exfoliator Products (eg. Products containing glycolic acid and salicylic acid)
|
To improve skin texture due to unaesthetic skin appearance caused by pigmentation, post acne scars, photo damage, etc.
NOTE : The ingredient and intended use should comply with the Guidelines for Control of Cosmetic Products in Malaysia. |
COSMETIC
|
NPRA |
|
|
24. |
Personal Care Products |
||||
|
NOTE : The product should be rinsed off. |
COSMETIC
|
NPRA |
||
|
DRUG
|
NPRA |
|||
|
Vaginal douching is the process of intravaginal cleansing with a liquid solution for : - personal hygiene or aesthetic reasons - preventing or treating/managing vaginal infections - symptomatic relief of minor vaginal soreness, irritation, itching - cleansing and deodorizing after menstruation - washing out vaginal medication, if so instructed by the physician - deodorizing and washing out the accumulations of normal secretions - removing contraceptive creams and jellies - cleansing the vaginal vault after sexual relations
NOTE : - Douching is not recommended during pregnancy - A douch is to be used as a cleanser and it should not be used as a contraceptive |
MEDICAL DEVICE (If it contains a pharmacologically active substance, it may be classified as DRUG)
|
MDA |
||
(eg. gel, foam, liquid) |
For general hand hygiene without therapeutic claims. |
COSMETIC
|
NPRA |
||
|
To use as a vaginal lubricant during the climaterium (pre-menopause, menopause, post-menopause) and to treat irritations in vaginal epithelium in cases of physiological decrease of lubrication and consequent increase in vaginal dryness. |
MEDICAL DEVICE (If it contains a pharmacologically active substance, it may be classified as DRUG)
|
MDA |
||
|
25. |
Skin Barrier Product (eg. lotion, emulsion, ointment, cream)
|
|
MEDICAL DEVICE (If it contains a pharmacologically active substance, it may be classified as DRUG)
|
MDA |
|
|
DRUG
|
NPRA |
|||
|
COSMETIC |
NPRA |
|||
|
26. |
Soft tissue filler/ Dermal filler |
To correct cutaneous contour deformities of the skin (e.g., moderate to severe facial wrinkles and folds such as nasolabial folds, scars), particularly in cases of aging or degenerative lesions.
|
MEDICAL DEVICE (If it incorporates an ancillary local anaesthetic eg. lidocaine, it will be classified as a Device-Drug combination product regulated as MEDICAL DEVICE) |
MDA
|
|
|
27. |
Synthetic fluid tissue reconstructive material |
As a submucosal implant in the urinary tract for urinary incontinence or vesicoureteral reflux.
It may also be injected into the vocal cords to treat the effects of paralysis, atrophy, or scarring. After application, this device cannot be reused.
|
MEDICAL DEVICE (If it incorporates an ancillary pharmacologically active substance eg. local anaesthetic such as lidocaine, it will be classified as a Device-Drug combination product regulated as MEDICAL DEVICE) |
||
|
28. |
Synovial joint replacement fluid (Joint lubricant) |
To help cushion the joint, especially in cases of reduced endogenous synovial fluid viscosity from degenerative disease. |
MEDICAL DEVICE |
MDA |
|
|
29. |
Wart Products (eg. pen applicator containing a caustic agent, cyryogenic kit with refrigerant) |
a. Containing a caustic agent eg. trichloroacetic acid (TCA) that destroys warts by chemical coagulation of proteins.
|
DRUG
NOTE : If a device component is present, it will be regulated on a case to case basis |
NPRA |
|
|
b. Cryotherapy which destroys warts by freezing them using a very cold substance eg. liquid nitrogen or refrigerant made from dimethyl ether and propane.
|
MEDICAL DEVICE |
MDA |
|||
|
30. |
Wound care/ treatment products |
||||
|
a. To administer a medicinal substance to the wound eg. antimicrobial/ antiseptic agent for the purpose of controlling infection. |
DRUG
|
NPRA |
||
|
b. To provide a protective layer/barrier to the wound and prevent microbial penetration and create healing environment. It may incorporate an ancillary medicinal substance eg. antimicrobial/ antiseptic agent. |
MEDICAL DEVICE |
MDA
|
|||
|
To facilitate the infiltration of native skin elements (e.g. fibroblasts, leukocytes, blood vessels) for skin regeneration. |
MEDICAL DEVICE |
MDA |
||
(e.g., abrasion, laceration, cut, ulcer) |
To facilitate local haemostasis. It is available in various forms (e.g., gel, spray, powder, ointment, plaster/gauze pad) that can be applied directly to the wound where it forms a seal of transparent layer. |
MEDICAL DEVICE |
MDA
|
||
|
To use as the wound covering material for deep body cavity to reduce the adhesion of surrounding tissues by applying to the surgical area
|
MEDICAL DEVICE |
MDA |
||
(eg. silver nitrate/ silver sulfadiazine/ colloidal silver gel, cream)
|
To administer/ apply an antiseptic to wounds with mild to moderate exudates such as: - First and second degree burns - Traumatic wounds - Surgical wounds - Partial full thickness wounds - Grafted wounds and donor sites - Lacerations and abrasions
|
DRUG |
NPRA |
||
|
An intravascular catheter securement device is a device with an adhesive backing that is placed over a needle or catheter and is used to keep the hub of the needle or the catheter flat and securely anchored to the skin. The antimicrobial agent provides ancillary antimicrobial activity to reduce skin colonization and catheter colonization, supress regrowth of microorganism’s, and reduce catheter-related bloodstream infections (CRBSI) in patients with central venous or arterial catheters.
|
DEVICE-DRUG combination product regulated as MEDICAL DEVICE |
MDA
|
||
Note:
- The above table is to be used as guidance for classification only.
- The registration/notification of products that have been classified must follow the requirements that have been set forth as follows:
i- Drug & Cosmetic – The registration/notification is in accordance with the requirements set forth in the Poisons Act 1952 and its Regulations, Sale of Drugs Act 1952 and the Control of Drugs and Cosmetics Regulations 1984.
ii- Medical Device – The registration is in accordance with the requirements set forth in the Medical Devices Act 2012 (Act 737).
- Medical Device will be regulated by MEDICAL DEVICE Authority.
- Drug & Cosmetic will be regulated by the NATIONAL PHARMACEUTICAL REGULATORY DIVISION, Ministry of Health Malaysia.
- Drug – Device Combination Product will be regulated according to the classification that has been made and by the relevant agencies.
Circular : Bil (21) dlm.BPFK/PPP/01/03 Jld. 3)

References :
-
http://npra.moh.gov.my/index.php/recent-updates/circular-directive
-
PEKELILING KRITERIA BARU PENGKELASAN PRODUK FOOD-DRUG INTERPHASE (FDI)









