-
Product name, dosage form and strength shall be entered. (e.g. X Brand Ivermectin Injection 10mg/ml)
-
Product name is defined as a name given to a product which may be either a proprietary name (an invented name); or a generic name (common name) or scientific name, together with a trade mark or the name of the manufacturer.
-
Product name shall not imply the following:
-
Tricky, confusive and against the law;
-
Scandalous and offensive;
-
Prejudicial;
-
Notorious.
-
-
Any product name which is the same or similar either in writing/ pronunciation, with the product name of an adulterated product or a product that has been revoked due to safety concerns is prohibited.
-
The invented name shall not be liable to confusion with the common name.
-
The generic name means the international non-proprietary name recommended by WHO (rINN), or if one does not exist, the usual approved name.
-
The product name shall be shown on the product labelling i.e. immediate label, outer unit carton, package insert and patient information leaflet (PIL).
-
Dosage form and strength of product would need to be entered as part of product name to allow for multiple dosage forms (e.g. tablet, capsule) and strengths (e.g. 200mg and 400mg) for any particular named (proprietary or generic) product.
-
If a product name is found similar to another registered product or any other name which deemed inappropriate by the Authority, NPRA reserves the rights to request for the change of the product name.
How to Access the Product Registration Application form in the QUEST 3+ System?
To access the Quest 3+ system, user need to use the URL as below:
https://quest3plus.bpfk.gov.my/front-end
Figure 1 (shown below) will appear and the user will need to key-in the following information:
- User Name: User ID
- Password: Enter Password.
- Click Login
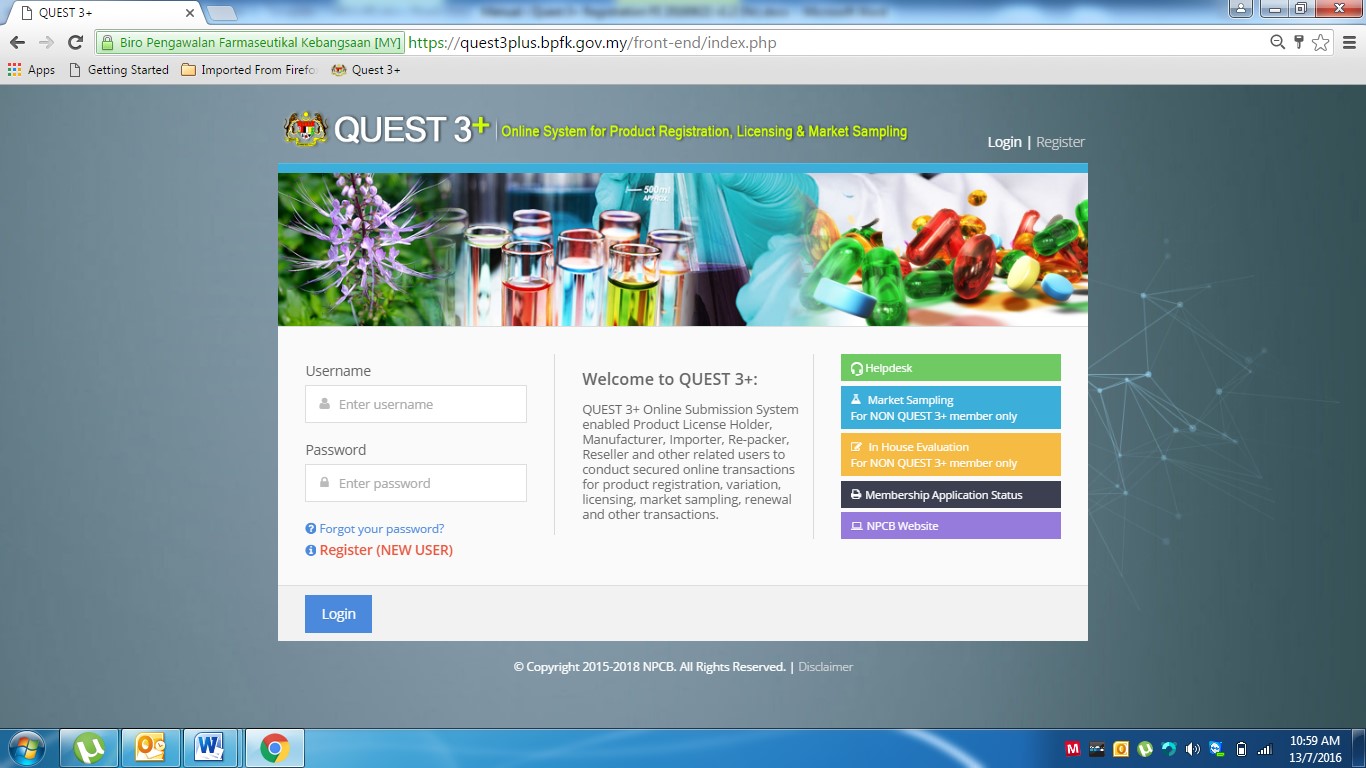
After successful log-in, Product Registration Application form can be accessed by clicking: Product Registration >> New Application Form >> Choose Product Category (Veterinary)








