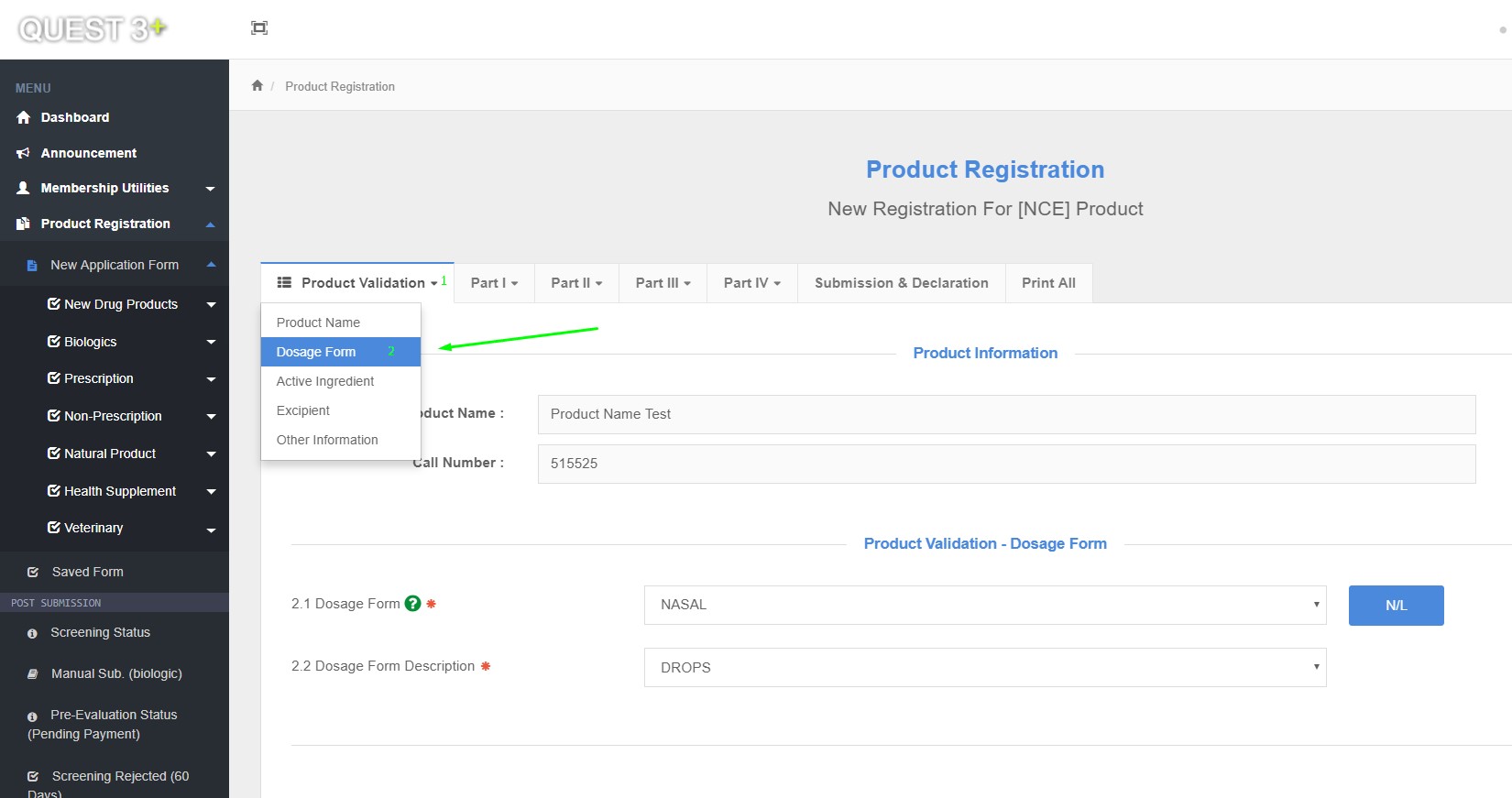
Dosage Form
2.1 Dosage Form
2.2 Dosage Form Description
2.3 Source of Capsule Shell
2.4 Certificate to verify the Source of Capsule Shell (attachment)
2.5 Colouring Agent used in Capsule Shell
2.6 Certificate of Analysis of Capsule Shell
- Please select dosage form and further select “in the form of” from the drop-down list.
- For example, a tablet may be in the form of chewable, coated (enteric, film, or sugar), uncoated, dispersible, effervescent, extended release, sublingual, etc.
- Tablets
- Caplet, Lozenge, Chewable tablet, Dispersible tablet, Effervescence tablet, uncoated tablet, enteric coated tablet, Sugar coated tablet, Film coated tablet, extended release tablet;
- Capsules
- Soft capsule, Hard capsule, Enteric coated capsule, Chewable soft capsule, Extended released capsule;
iii. Powder/ Granules;
- Liquid
- Emulsion, syrup, spray, suspension.
- The form that correctly describes it in terms of its product quality control specifications and performance shall be selected.
Note: A separate application for registration is required for each dosage form.
How to Acces Dossage Form in QUEST System ?
Product Registration >> New Application Form >> Product Validation >> Dosage Form