Registering for the QUEST3+ System is straightforward, and you do not need to hire a regulatory consultant or agent. Follow the 6 simple steps below to complete your registration:
STEPS to register as a user for the QUEST 3+ System:
1. Register as a Pre-Membership user to access the Full Membership Application Form.You can register HERE.
2. After verify your email, login to Pre-membership module and click on the "QUEST3+ Full Membership Application Form" (as shown in the screenshot below).
3. Fill all the required information on the registration form.
4. NPRA Officer will review your application, and you will be notified by email of the approval or rejection of your membership request.
5. Once the application approved, complete remaining steps at https://www.msctrustgate.com/mytrustid/enrollment?q=quest3plus
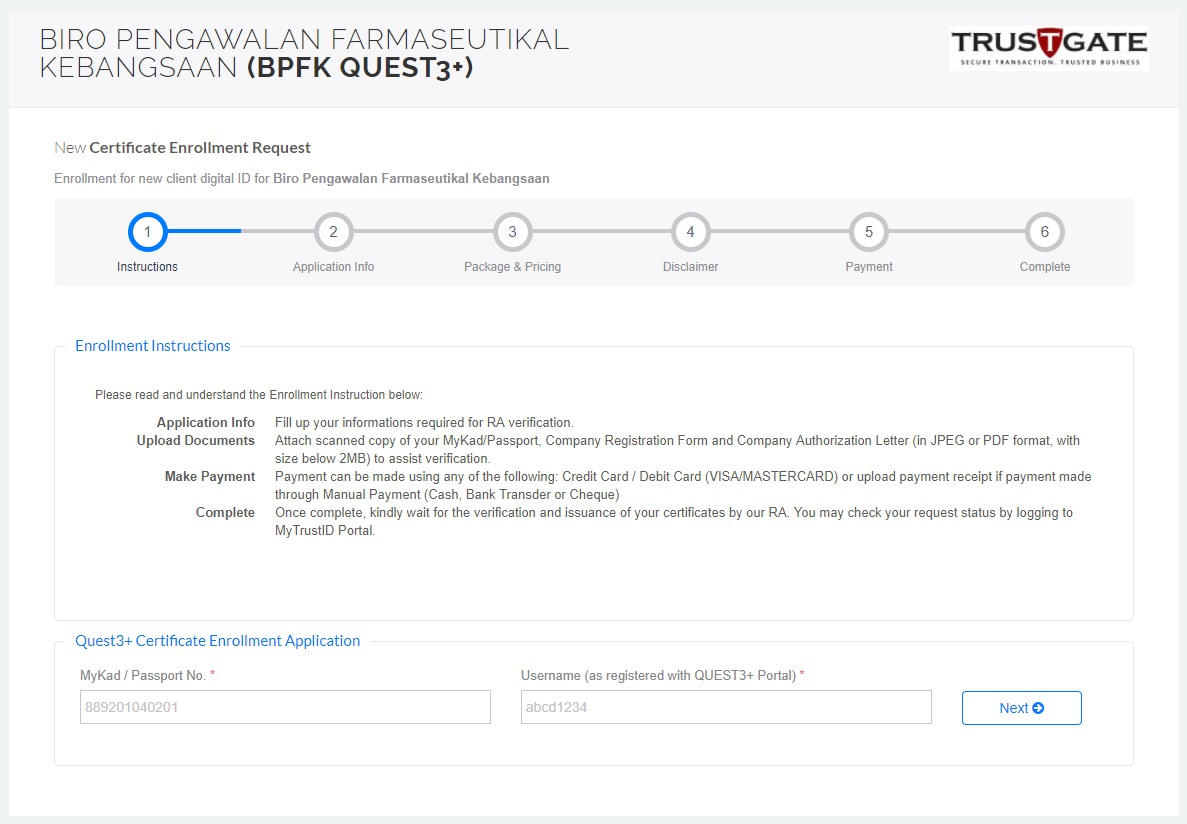
6.Payment completed
Upon completion of these 5 EASY STEPS, the USB Token can be retrieved by walk-in to MSC Trustgate (before 3pm) or postal delivery (will take up to 7 working days).
If the USB token is not received within 7 working days, kindly contact MSC Trustgate.com Sdn. Bhd. at 03-8318 1800 or email to This email address is being protected from spambots. You need JavaScript enabled to view it..
MSC Trustgate.com Sdn. Bhd.
Suite 2-9,Level 2, Block 4801
CBD Perdana, Jalan Perdana
63000 Cyberjaya
Selangor Darul Ehsan
After getting token, you can begin to configure your PC/laptop by referring QUEST3+ User Manual
Following are the 3 important links that can assist your product registration process:
2. FAQ
3. Drug Registration Guideline / Cosmetic Registration Guideline
If you have any enquiries and need assistance on registration process, you can :
1. Submit queries at Public Enquiry form
2. OR call Quest Information Unit at 03-7883 5400 (select option for Technical Quest)
*NPRA does not appoint nor endorse any third parties (consultant, agent, etc.) for doing regulatory works on behalf of applicants. NPRA will not be held liable for any statements nor activities conducted and reflected by these third parties. NPRA will not be held accountable for any liabilities resulting from such practices.










